Figure 1. Suggested model of impaired tamoxifen treatment in the presence of HER4 receptor expression and activation and conceivable therapeutic interventions.
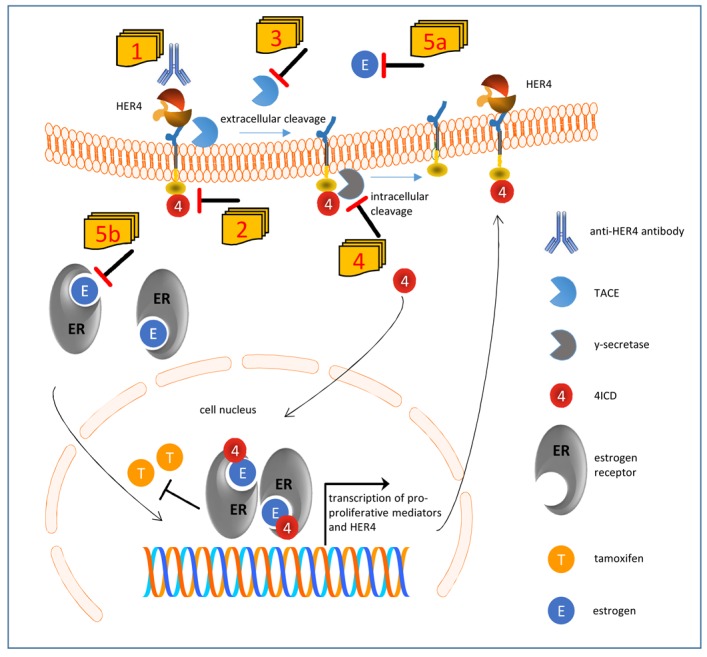
HER4 can be processed by a two-step proteolytic activation. First tumor necrosis factor α converting enzyme (TACE) cleaves the extracellular domain, and subsequently y-secretase cleaves the intracellular domain of HER4 (4ICD) which is released into inner cell compartments. If translocated into the nucleus, 4ICD as an ER co-activator enhances the pro-proliferative effects of estrogen. Within an autoloop, 4ICD also enhances the transcription of HER4 itself. However, the 4ICD also impairs the efficient inhibition of ER by tamoxifen. The unfavorable activity of HER4 can be potentially abolished by a number of strategies: HER4 activity and cleavage can be blocked by a specific anti-HER4 antibody [1] or by an HER4 kinase inhibitor [2]. Alternatively the extracellular and intracellular cleavage can be blocked by an inhibitor of TACE [3] or γ-secretase [4], respectively. Finally, the sequestration of estradiol [5a] would also impair the ligand independent but TACE induced cleavage of HER4 and would additionally attenuate the activation of the ER [5b].
