Abstract
Background
Numerous medications are available for the acute treatment of migraine in adults, and some have now been approved for use in children and adolescents in the ambulatory setting. A systematic review of acute treatment of migraine medication trials in children and adolescents will help clinicians make evidence‐informed management choices.
Objectives
To assess the effects of pharmacological interventions by any route of administration versus placebo for migraine in children and adolescents 17 years of age or less. For the purposes of this review, children were defined as under 12 years of age and adolescents 12 to 17 years of age.
Search methods
We searched seven bibliographic databases and four clinical trial registers as well as gray literature for studies through February 2016.
Selection criteria
We included prospective randomized controlled clinical trials of children and adolescents with migraine, comparing acute symptom relieving migraine medications with placebo in the ambulatory setting.
Data collection and analysis
Two reviewers screened titles and abstracts and reviewed the full text of potentially eligible studies. Two independent reviewers extracted data for studies meeting inclusion criteria. We calculated the risk ratios (RRs) and number needed to treat for an additional beneficial outcome (NNTB) for dichotomous data. We calculated the risk difference (RD) and number needed to treat for an additional harmful outcome (NNTH) for proportions of adverse events. The percentage of pain‐free patients at two hours was the primary efficacy outcome measure. We used adverse events to evaluate safety and tolerability. Secondary outcome measures included headache relief, use of rescue medication, headache recurrence, presence of nausea, and presence of vomiting. We assessed the evidence using GRADE (Grading of Recommendations Assessment, Development and Evaluation) and created 'Summary of findings' tables.
Main results
We identified a total of 27 randomized controlled trials (RCTs) of migraine symptom‐relieving medications, in which 9158 children and adolescents were enrolled and 7630 (range of mean age between 8.2 and 14.7 years) received medication. Twenty‐four studies focused on drugs in the triptan class, including almotriptan, eletriptan, naratriptan, rizatriptan, sumatriptan, sumatriptan + naproxen sodium, and zolmitriptan. Other medications studied included paracetamol (acetaminophen), ibuprofen, and dihydroergotamine (DHE). More than half of the studies evaluated sumatriptan. All but one study reported adverse event data. Most studies presented a low or unclear risk of bias, and the overall quality of evidence, according to GRADE criteria, was low to moderate, downgraded mostly due to imprecision and inconsistency. Ibuprofen was more effective than placebo for producing pain freedom at two hours in two small studies that included 162 children (RR 1.87, 95% confidence interval (CI) 1.15 to 3.04) with low quality evidence (due to imprecision). Paracetamol was not superior to placebo in one small study of 80 children. Triptans as a class of medication were superior to placebo in producing pain freedom in 3 studies involving 273 children (RR 1.67, 95% CI 1.06 to 2.62, NNTB 13) (moderate quality evidence) and 21 studies involving 7026 adolescents (RR 1.32, 95% CI 1.19 to 1.47, NNTB 6) (moderate quality evidence). There was no significant difference in the effect sizes between studies involving children versus adolescents. Triptans were associated with an increased risk of minor (non‐serious) adverse events in adolescents (RD 0.13, 95% CI 0.08 to 0.18, NNTH 8), but studies did not report any serious adverse events. The risk of minor adverse events was not significant in children (RD 0.06, 95% CI − 0.04 to 0.17, NNTH 17). Sumatriptan plus naproxen sodium was superior to placebo in one study involving 490 adolescents (RR 3.25, 95% CI 1.78 to 5.94, NNTB 6) (moderate quality evidence). Oral dihydroergotamine was not superior to placebo in one small study involving 13 children.
Authors' conclusions
Low quality evidence from two small trials shows that ibuprofen appears to improve pain freedom for the acute treatment of children with migraine. We have only limited information on adverse events associated with ibuprofen in the trials included in this review. Triptans as a class are also effective at providing pain freedom in children and adolescents but are associated with higher rates of minor adverse events. Sumatriptan plus naproxen sodium is also effective in treating adolescents with migraine.
Plain language summary
Drugs for the acute treatment of migraine in children and adolescents
Background and review question
Migraine is a painful and debilitating disorder that is common in children (under 12 years of age) and adolescents (12 to 17 years of age). Common symptoms reported during a migraine attack are headache, nausea, vomiting, and sensitivity to light and sound. Many treatments for migraine are available, of which the most common are paracetamol (also known as acetaminophen), ibuprofen and other anti‐inflammatories, and triptans. Not all triptan medications are approved for use in children or adolescents, and approvals vary from country to country.
Study characteristics
In our review, we looked at 27 randomized controlled trials of drugs compared to placebo to find out which treatments were effective at providing pain freedom two hours after treatment. We also wanted to know what side effects might be caused by the treatments. A total of 7630 children received medication in the studies. The evidence is current to February 2016. Each study had between 13 and 888 participants. Their average age was 12.9 years and ranged from 8.2 to 14.7 years. Nineteen of the studies were funded by the drug manufacturer.
Key results
Ibuprofen appears to be effective in treating children with migraine, but the evidence is limited to only two small trials. Ibuprofen is readily available and inexpensive, making it an excellent first choice for migraine treatment. Paracetamol was not shown to be effective in providing pain freedom in children, but we only found one small study. Triptans are a type of medication designed specifically to treat migraine and are often effective at providing greater pain freedom in children and adolescents. The triptans examined in children included rizatriptan and sumatriptan, while almotriptan, eletriptan, naratriptan, rizatriptan, sumatriptan, and zolmitriptan were examined in adolescents. The combination of sumatriptan plus naproxen sodium is also effective at treating adolescents with migraine. Overall, there is a risk that the triptan medications may cause minor unwanted side effects like taste disturbance, nasal symptoms, dizziness, fatigue, low energy, nausea, or vomiting. The studies did not report any serious side effects.
Quality of the evidence
The overall quality of the evidence provided by the review was moderate for the triptans, but low for paracetamol and ibuprofen, as we only identified a few studies. More studies need to look at the effects of each of the migraine treatments in children and adolescents separately.
Summary of findings
Background
Migraine is a common and disabling disease, affecting 3% to 10% of children and adolescents (Stovner 2007). Children as young as two years of age may be affected, and most adults with migraine have their first headache in early childhood or adolescence (Bille 1997). Quality‐of‐life studies indicate that migraine has a significant negative impact on a child (Powers 2003; Powers 2004). Indeed, migraines can be a tremendous source of anxiety for children, adolescents, and their parents, disrupting both school obligations and parental work responsibilities. These concerns may be amplified when there is uncertainty on the physician's part as to the best treatment.
The International Classification of Headache Disorders, 3rd edition, beta version (ICHD‐3 beta), provides the most accepted and current definition of migraine. The presence of headache, often associated with nausea, vomiting, or both is common to adults, adolescents, and children with migraine. However, the criteria acknowledge that children and adolescents' migraines may be shorter but will last at least two hours, and the pain may be bilateral over the frontotemporal head regions or non‐pulsatile. Adolescents, like adults, will usually begin to report unilateral pulsatile pain. The presence of photophobia and phonophobia may need to be inferred from behaviour such as a preference for a quiet and dimly lit room during a migraine attack.
Treatments for migraine include symptom‐relieving and preventive strategies. Preventive medications are used to reduce the frequency and severity of migraine attacks. Symptom‐relieving therapies commonly aim to eliminate head pain and reduce the symptoms associated with migraine, including nausea, phonophobia, and photophobia. Oral analgesics such as paracetamol and ibuprofen are the mainstay of acute therapy for migraine in children and adolescents (Hämäläinen 2002). However, other agents such as ergot derivatives (e.g. dihydroergotamine) and the serotonin 1b/1d receptor agonists (triptans) have demonstrated efficacy in adults. Many of these medications have now been studied in children and adolescents, and some are approved for use in the pediatric age group. A practice parameter on this topic has been published by the American Academy of Neurology Quality Standards Subcommittee and the Practice Committee of the Child Neurology Society (Lewis 2004). This systematic review will summarize and update the evidence base and provide a meta‐analysis of the data.
Objectives
To assess the effects of pharmacological interventions by any route of administration versus placebo for migraine in children and adolescents 17 years of age or less. For the purposes of this review, children were defined as under 12 years of age and adolescents 12 to 17 years of age.
Methods
Criteria for considering studies for this review
Types of studies
We included all prospective, placebo‐controlled trials of pharmacological interventions for symptomatic or acute treatment of migraine in children and adolescents in the outpatient setting if allocation to treatment groups was randomized (see Differences between protocol and review). We included studies regardless of design (i.e. parallel‐group or cross‐over), publication status, or language of publication. We included cross‐over studies, as migraine is an episodic disorder, and we did not expect any carry‐over or period effects (see Differences between protocol and review). We excluded non‐placebo‐controlled studies, concurrent cohort comparisons and other quasi‐ or non‐experimental designs.
Types of participants
We included studies involving pediatric participants 17 years of age or less with a diagnosis of migraine with or without aura. For the purposes of the review, we defined children as under 12 years of age and adolescents as 12 to 17 years of age (see Differences between protocol and review). We recorded inclusion age criteria, including median and mean age of subjects. We excluded studies involving both pediatric and adult patients unless they reported results separately for the pediatric patients. We analyzed separately the data from studies that included both children and adolescents when possible. If studies did not report the data separately, we used the mean age as a surrogate. If the mean age in the study was less than 12 years, we considered the study population to be of predominantly childhood age. If the mean age was greater than or equal to 12 years, we considered the study population to be of predominantly adolescent age.
Migraine is defined by clinical symptoms and signs in the 3rd edition of the International Classification of Headache Disorders, beta version (ICHD‐3 beta). ICHD‐3 beta includes revised comments for the diagnosis of migraine in children and adolescents, including shorter duration of headache (2 to 72 hours), bilateral frontotemporal location, and the presence of photophobia and phonophobia as inferred from behaviour. There have been two other versions of the International Classification of Headache Disorder and a proposed revision of the 1988 criteria in the context of children or adolescents (IHS 1988; ICHD‐2; Winner 1995). We included a study in this review if investigators used any version of the International Headache Society classification systems above or the proposed revision for pediatrics for the diagnosis of migraine with or without aura.
Types of interventions
We included studies allocating participants to receive a pharmacological intervention by any route of administration for symptomatic acute treatment of a migraine attack. Acceptable comparator groups included placebo or other active drug treatments. We identified the use of preventive medication and examined this in subgroup analysis, but discontinuation was not required for inclusion.
Types of outcome measures
We chose outcomes to assess both efficacy and safety. We selected the primary efficacy outcome based on the suggested guidelines for controlled trials of drugs in migraine (Tfelt‐Hansen 2012). The primary outcome for safety was based on the report of adverse events. All outcome measures were reported for the treatment of a single attack (see Differences between protocol and review).
Primary outcomes
The primary outcome measure for efficacy was the percentage of pain‐free participants at two hours; we defined pain freedom as the absence of pain at two hours before the use of additional or rescue medication (see Differences between protocol and review). Headache relief is a frequently used primary outcome measure that is variably defined based on the scale used to assess pain. For example, if using the four level scale of none, mild, moderate, or severe pain, investigators or physicians may define headache relief as a decrease in pain from moderate or severe to mild or none. Some in the migraine research community have challenged the use of headache relief as a primary outcome measure; for example, migraine sufferers generally do not consider headache relief a success and expect pain freedom from an intervention (Davies 2000; Lipton 2002). Given these considerations, we classified headache relief as a secondary outcome measure (see Differences between protocol and review).
We used the percentage of participants with any adverse event(s) as the primary safety outcome measure, and this was required for inclusion in the analysis (see Differences between protocol and review). We defined adverse events as any unwanted effect that occurred during treatment. We also documented the proportion of participants reporting any serious adverse events when possible. Serious adverse events included death, any life‐threatening condition, hospitalization, disability or permanent damage, required intervention to prevent permanent damage, or any other important medical event that could jeopardize the participant or require medical or surgical intervention.
Some aspects of the original protocol were not necessary to implement given the way studies reported outcome data. We document these considerations in the Differences between protocol and review section.
Secondary outcomes
We assessed the following secondary outcome measures.
Headache relief (headache response): the percentage of participants with headache relief at two hours is typically defined as a decrease in headache intensity from severe or moderate to mild or none at two hours prior to the use of rescue medication. When studies used alternate definitions of pain intensity (e.g. numerical scale), they needed to describe a level of relief that would be meaningful to a participant and to reflect a decrease in headache intensity similar to that assumed in the above definition.
Rescue medication: the percentage of participants taking rescue medication at two hours or earlier to a maximum of six hours after the test drug. The definition of rescue medication was variable and often included any use of medication to treat the recurrence of headache within a specified timeframe (i.e. usually 24 hours). For the purposes of the review, rescue medication was defined as the use of any medication between 2 and 24 hours.
Headache recurrence: the percentage of participants who were initially pain‐free or achieved the study primary outcome of headache relief within 2 hours without the use of rescue medication but who experienced recurrence of any headache from 2 to 48 hours.
Presence of nausea: percentage of participants with nausea at two hours after taking the test drug.
Presence of vomiting: percentage of participants with vomiting within two hours of taking the test drug.
We did not include participant preference, presence of photophobia, or presence of phonophobia in the analysis as originally planned in the protocol (see Differences between protocol and review).
Search methods for identification of studies
We conducted a search of electronic databases in collaboration with a research librarian using search strategies to identify the highest level of evidence for the topic. In addition, we manually searched other sources listed below.
Electronic searches
We systematically searched the following databases:
Cochrane Central Register of Controlled Trials (CENTRAL) (1991 to 2013, Issue 3).
OvidSP MEDLINE (1946 to February 2016).
Ovid MEDLINE In‐Process & Other Non‐Indexed Citations (2012 to February 2016).
EMBASE (1980 to February 2016).
Database of Abstracts and Reviews of Effects (1991 to April 2013).
International Pharmaceutical Abstracts (1970 to April 2013).
PsycINFO (1806 to April 2013).
EBSCOhost CINAHL (Cumulative Index of Nursing and Allied Health) (1937 to April 2013).
The search strategies used a combination of text words and medical subject headings (MeSH), adapted for each database searched: concepts included migraine, headache, cephalgia or cephalalgia, drug therapy, drug treatment, antimigraine therapy, antimigraine treatment, and treatment outcome, combined with drugs and acute treatments known to be used for migraine in children and adolescents. These terms were combined with a pediatric filter designed by the librarian (Lisa Tjosvold) of the Cochrane Child Health Field.
Complete search strategies are given in Appendix 1.
Searching other resources
We conducted a gray literature search including reviewing the reference lists of included studies and handsearching meeting abstracts from the American Headache Society and International Headache Society Scientific meetings. The review authors attempted to contact primary authors, experts in the area, and drug manufacturers (GlaxoSmithKline, AstraZeneca, Ortho‐McNeil, Merck, and Pfizer) for information on recent, ongoing, or unpublished trials. We searched ClinicalTrials.gov for new or ongoing studies and used Current Controlled Trials (www.controlled-trials.com) to search across multiple trial registries. GlaxoSmithKline (www.gsk-clinicalstudyregister.com) and AstraZeneca (www.astrazenecaclinicaltrials.com) have clinical trial registries and report on both published and unpublished studies.
Data collection and analysis
Selection of studies
We identified potentially relevant articles by reviewing the titles and abstracts from the original search. We considered studies with insufficient information in the title or abstract as potentially relevant articles for further assessment. We then reviewed the full text of potentially relevant studies for inclusion or exclusion. Two reviewers independently carried out both steps. A third, independent reviewer resolved any disagreements that arose between them.
Data extraction and management
One reviewer used a standardized data abstraction form to extract data, which a second reviewer then checked for accuracy and completeness. We recorded extracted data in Review Manager 5 (RevMan 2014). A third, independent reviewer resolved discrepancies.
Assessment of risk of bias in included studies
Two reviewers assessed risk of bias using the 'Risk of bias' table for each study. Reviewers assessed five domains before making a judgement: sequence generation, allocation concealment, blinding, incomplete outcome data, and selective reporting (Higgins 2011). We resolved any disagreements by discussion. We did not use the Jadad scale as planned in the protocol, as per current recommendations of the Cochrane Collaboration (Differences between protocol and review).
Assessment of quality of evidence in included studies
We assessed the overall quality of the evidence for each outcome using the GRADE system and included our assessment in the 'Summary of findings' tables in order to present the main findings of the review in a transparent and simple tabular format (GRADEPro 2015). In particular, we included key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the main outcomes.
The GRADE system uses the following criteria for assigning a level of evidence.
High: further research is very unlikely to change our confidence in the estimate of effect.
Moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low: any estimate of effect is very uncertain.
We decreased the grade if we found:
serious (− 1) or very serious (− 2) limitations to study quality;
important inconsistency(s) (− 1);
some (− 1) or major (− 2) uncertainty about directness;
imprecise or sparse data (− 1);
high probability of reporting bias (− 1).
'Summary of findings' table
We decided post hoc to include four 'Summary of findings' tables to present the main findings in a transparent and simple tabular format. In particular, we included key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes pain freedom at 2 hours and adverse events, for the four main comparisons.
Measures of treatment effect
We reported the risk ratio (RR) for all primary and secondary efficacy outcome measures with 95% confidence intervals (CIs) as well as the number needed to treat for an additional beneficial outcome (NNTB). We reported the risk difference (RD) with 95% CIs for the adverse events as the primary measure of harm as well as the number needed to treat for an additional harmful outcome (NNTH).
Unit of analysis issues
We included cross‐over trials with binary outcomes in the analysis (Curtin 2002). None of the cross‐over trials reported any significant carry‐over or period effects, and none would be expected based on the acute episodic nature of migraine.
Dealing with missing data
We only analyzed the available data for all outcomes. We assessed the possibility of missing studies as described below (see Assessment of reporting biases). We considered variability in the reporting of outcomes to be random.
Assessment of heterogeneity
We assessed heterogeneity using the I2 statistic. We interpreted the I2 statistic as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of reporting biases
We assessed reporting biases qualitatively by visually examining the funnel plot and quantitatively using a modified version of Egger's test (Egger 1997; Harbord 2006), as implemented in the metabias module for Stata statistical software package on the triptan versus placebo studies in adolescents using the pain‐free outcome (Stata 14).
Data synthesis
We pooled studies using a Mantel‐Haenszel, random‐effects model. We pooled dichotomous outcomes, and calculated RRs with 95% CIs. We calculated the number needed to treat for an additional beneficial outcome (NNTB) for RRs of the primary outcome that were statistically significant. For adverse events, we combined data combined using RDs with 95% CIs. We calculated the number needed to treat for an additional harmful outcome (NNTH) for RDs of adverse events that were statistically significant. We included studies with small sample sizes and imprecise effect estimates, but we graded them lower for quality and weighted them accordingly in the meta‐analyses.
We combined trials of cross‐over design with parallel group trials in the meta‐analysis. We included outcome measures from each intervention period in the analysis as if the trial were a parallel group trial. We also included three‐way cross‐over studies with two intervention periods and one placebo period as if they were a parallel group trial, but we divided the placebo period in two to avoid double counting the placebo interventions. Paired analysis was not possible for any of the included studies (see Differences between protocol and review). This method of including cross‐over studies will tend to produce wider confidence intervals and is considered a more conservative assessment of treatment effect given that these studies will receive a lower weight in the meta‐analyses. We included study design in the sensitivity analysis to assess the influence of cross‐over design on our final conclusions.
Subgroup analysis and investigation of heterogeneity
We used the I2 statistic to determine the presence of heterogeneity and test for subgroup differences as implemented in RevMan, used to prepare this review (Higgins 2002; RevMan 2014). We performed all subgroup analyses for the pain‐free outcome measure using the placebo‐controlled studies of triptan medications versus placebo in adolescents (except for the age‐based subgroup analysis).
Post hoc subgroup analyses included the following.
Intranasal route.
Preventive medication permitted during the study.
Sensitivity analysis
We examined potential sources of heterogeneity using the following a priori sensitivity analyses (see Differences between protocol and review).
Allocation concealment (low, unclear, or high risk of bias).
Cross‐over versus parallel‐group study design.
Source of funding (pharmaceutical, non‐pharmaceutical, or unclear).
Reported in a peer‐reviewed indexed journal.
Small sample size ≤ 50.
We performed all sensitivity analyses for the pain‐free primary outcome using the studies of triptan medications versus placebo in adolescents.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies, Characteristics of ongoing studies.
Results of the search
The literature search and review of the gray literature yielded 15,811 unique citations, 379 of which we assessed as full‐text articles for eligibility. Some of our data requests to manufacturers were met with referrals to trial registry websites or data were not made available. A total of 27 randomized placebo‐controlled trials of acute drug therapy for migraine met our inclusion criteria (Figure 1).
1.
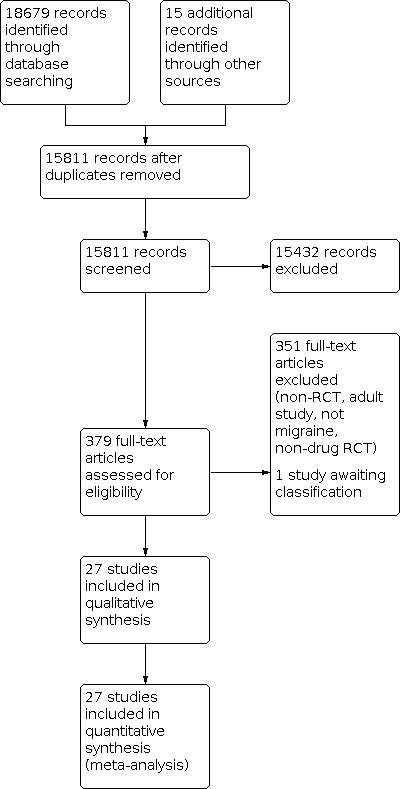
Study flow diagram.
Included studies
The mean age of inclusion was 12.9 years with a range of means between 8.2 and 14.7 years. The minimum age of inclusion was 4 years in one study (Hämäläinen 1997a), and the maximum age for inclusion was 18 years in two studies (Evers 2006; Hämäläinen 1997b). Three studies included only children under 12 (Hämäläinen 2002; Lewis 2002; Ueberall 1999), and one study reported data for children and adolescents separately (Ho 2012). Six studies included children and adolescents, but they did not report the data separately (Ahonen 2004; Ahonen 2006; Evers 2006; Hämäläinen 1997a; Hämäläinen 1997b; Hämäläinen 1997c). Participants in two of the studies reporting children and adolescents combined had a mean age of less than 12 years and were included in the analysis as studies of predominantly children (Hämäläinen 1997a; Hämäläinen 1997c). Participants in the remaining four studies where children and adolescents were combined had a mean age of greater than 12 years, and we considered them to be studies of predominantly adolescents for the analysis. Finally, 17 studies included only adolescents over the age of 12 years (Callenbach 2007; Derosier 2012; Fujita 2014; Lewis 2007; Linder 2008; NCT01211145; Rothner 1997; Rothner 1999a; Rothner 1999b; Rothner 1999c; Rothner 2006; Visser 2004a; Winner 1997; Winner 2000; Winner 2002; Winner 2006; Winner 2007). The mean number of participants randomized was 359 with a range of 13 to 888. We summarize the characteristics of included studies in Table 5, and we summarize the individual studies in Table 6. We provide more details, including a risk of bias assessment, in Characteristics of included studies.
1. Summary of characteristics of included studies.
| Study characteristics | Criteria | N= 27a | % |
|
Study design |
Parallel | 16 | 59% |
| Cross‐over | 11 | 41% | |
|
Sponsorship |
Pharmaceutical | 19 | 70% |
| Non‐pharmaceutical | 5 | 19% | |
| Unclear | 3 | 11% | |
|
Age inclusion criteria |
Adolescents (12‐17 years) | 17 | 63% |
| Children and adolescents | 6 | 22% | |
| Children (< 12 years) | 4 | 15% | |
|
Pain scale |
4‐point scale | 21 | 78% |
| 5‐faces scale | 5 | 18% | |
| VAS | 1 | 4% | |
|
Preventive medication permitted? |
Yes | 9 | 33% |
| No | 13 | 48% | |
| Unclear | 5 | 19% | |
|
Route of delivery |
Oral | 19 | 70% |
| Intranasal | 8 | 30% | |
|
Medications |
Paracetamol | 1 | 4% |
| Ibuprofen | 3 | 7% | |
| Triptans | 24 | 85% | |
| DHE | 1 | 4% | |
|
Triptan medications |
Almotriptan | 1 | 4% |
| Eletriptan | 1 | 4% | |
| Naratriptan | 1 | 4% | |
| Rizatriptan | 4 | 17% | |
| Sumatriptan | 12 | 50% | |
| Sumatriptan + naproxen sodium | 1 | 4% | |
| Zolmitriptan | 4 | 17% |
DHE: dihydroergotamine; VAS: visual analogue scale.
aThe total number of studies listed in Medications does not add up to 27 as two studies (Hämäläinen 1997a & Evers 2006) compared multiple medications.
2. Summary table of included studies.
| Review Manager ID | Study design | Agent | Route |
Child. (< 12 yrs) |
Adolesc. (12‐17 yrs) |
Mean age | No | % Female |
| Paracetamol | ||||||||
| Hämäläinen 1997a | Cross‐over | — | PO | Yes | Yes | 10.7 | 80 | 50% |
| Ibuprofen | ||||||||
| Hämäläinen 1997a | Cross‐over | — | PO | Yes | Yes | 10.7 | 78 | 50% |
| Lewis 2002 | Parallel | — | PO | Yes | No | 9.0 | 84 | ND |
| Evers 2006 | Cross‐over | — | PO | Yes | Yes | 13.9 | 29 | 56% |
| Triptans (< 12 years) | ||||||||
| Ho 2012 | Parallel | Rizatriptan | PO | Yes | NA | ND | 200 | 44% |
| Ueberall 1999 | Cross‐over | Sumatriptan | IN | Yes | No | 8.2 | 14 | 50% |
| Hämäläinen 2002 | Cross‐over | Sumatriptan | IN | Yes | No | 9.7 | 59 | 54% |
| Triptans (12‐17 years) | ||||||||
| Linder 2008 | Parallel | Almotriptan | PO | No | Yes | 14.4 | 714 | 60% |
| Winner 2007 | Parallel | Eletriptan | PO | No | Yes | 14.0 | 274 | 57% |
| Rothner 1997 | Parallel | Naratriptan | PO | No | Yes | 14.3 | 300 | 54% |
| Ho 2012 | Parallel | Rizatriptan | PO | NA | Yes | ND | 570 | 61% |
| Visser 2004a | Parallel | Rizatriptan | PO | No | Yes | 14.2 | 476 | 55% |
| Winner 2002 | Parallel | Rizatriptan | PO | No | Yes | 14.0 | 296 | 54% |
| Ahonen 2006 | Cross‐over | Rizatriptan | PO | Yes | Yes | 12.0 | 116 | 54% |
| Callenbach 2007 | Cross‐over | Sumatriptan | IN | No | Yes | 13.6 | 46 | 78% |
| Rothner 1999b | Parallel | Sumatriptan | PO | No | Yes | 13.6 | 92 | 52% |
| Winner 2000 | Parallel | Sumatriptan | IN | No | Yes | 14.1 | 507 | 52% |
| Hämäläinen 1997b | Cross‐over | Sumatriptan | PO | Yes | Yes | 12.3 | 23 | 52% |
| Rothner 1999c | Parallel | Sumatriptan | PO | No | Yes | 13.5 | 102 | 42% |
| Fujita 2014 | Parallel | Sumatriptan | PO | Yes | Yes | 14.1 | 144 | 58% |
| Winner 1997 | Cross‐over | Sumatriptan | PO | No | Yes | 13.9 | 298 | 58% |
| Winner 2006 | Parallel | Sumatriptan | IN | No | Yes | 14.3 | 731 | 55% |
| Ahonen 2004 | Cross‐over | Sumatriptan | IN | Yes | Yes | 12.4 | 94 | 46% |
| Rothner 1999a | Parallel | Sumatriptan | PO | No | Yes | 14.1 | 273 | 57% |
| Derosier 2012 | Parallel | Sumatriptan and Naproxen Sodium |
PO | No | Yes | 14.7 | 490 | 59% |
| Lewis 2007 | Cross‐over | Zolmitriptan | IN | No | Yes | 14.2 | 171 | 57% |
| Evers 2006 | Cross‐over | Zolmitriptan | PO | Yes | Yes | 13.9 | 29 | 56% |
| Rothner 2006 | Parallel | Zolmitriptan | PO | No | Yes | 14.2 | 696 | 59% |
| NCT01211145 | Parallel | Zolmitriptan | IN | No | Yes | 14 | 584 | ND |
| Dihydroergotamine | ||||||||
| Hämäläinen 1997c | Cross‐over | — | PO | Yes | Yes | 10.3 | 13 | 38% |
IN: intranasal; NA: not applicable; ND: no data available; No: total number in efficacy analysis (intention‐to‐treat when available); PO: per os (by mouth).
Excluded studies
We excluded two studies, one that compared intravenous prochlorperazine versus ketorolac in children and adolescents presenting to the Emergency Department (ED) (Brousseau 2004) and one that compared intravenous metoclopramide to placebo in children and adolescents presenting to the ED (NCT00355394) from the analysis as our review is focused on outpatient acute drug therapy. All other excluded studies were either not controlled or were non‐drug clinical trials (see Characteristics of excluded studies).
Awaiting classification
A single study was recently published and is awaiting classification (Winner 2015). It is a cross‐over study of sumatriptan + naproxen sodium in adolescents (see Characteristics of studies awaiting classification).
Risk of bias in included studies
We illustrated the risk of bias in included studies in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Investigators described all studies as randomized (low risk of selection bias (random sequence generation)), but the method of randomization was unclear in 19 studies (unclear risk of bias). Authors frequently employed generic descriptions of sequence generation such as 'randomized 1:1' or 'block randomization to two age groups'. Eight studies adequately reported allocation concealment, and we judged them to be at low risk of selection bias (allocation concealment). We judged the remaining 19 studies to be at unclear risk of bias for this domain.
Blinding
Generally, authors described all studies as double‐blind, but 17 studies did not report their methods for blinding clearly (unclear risk of bias). All studies were either an oral or intranasal medication compared with placebo. Studies seldom described efforts to match for taste, color, smell, etc. We considered 10 studies to be at low risk of bias.
Incomplete outcome data
We considered incomplete reporting of outcome data to confer a high risk of bias in Winner 2002 and an unclear risk in Hämäläinen 1997c. We considered the remaining 25 studies to be at low risk.
Selective reporting
Six studies were reported only in the sponsors' clinical trial report registry and published only in abstract form (Hämäläinen 2002; Rothner 1997; Rothner 1999a; Rothner 1999b; Rothner 1999c; Winner 1997), while one study was reported only in the sponsor's clinical trial report registry (NCT01211145). All included studies reported the pain‐free primary efficacy outcome. The reports available through the sponsors' clinical trial registries were comprehensive in reporting all planned outcome measures and greatly enhanced the reported data that was published in abstract form. Also, there were no discrepancies between published abstracts and the reports released through the sponsor's clinical trial registry. Derosier 2012 was the only study that did not report the headache relief secondary outcome, and Lewis 2002 did not report adverse event data; we judged these two studies to be at unclear risk of bias for this domain. We judged the remaining 25 studies to be at low risk of bias.
Other potential sources of bias
We assessed publication bias based on the pain‐free outcome for all triptans versus placebo in adolescents. On visual inspection, the funnel plot showed some asymmetry (Figure 4), suggesting the possibility of publication or other sources of bias and small‐study effects. We used Egger's test to explore small‐study effects, which were not significant (P = 0.139). The inclusion of published and unpublished data from the clinical trial registries would suggest a reduced risk of publication bias, as many of these studies were negative.
4.

Funnel plot of comparison: 7 Triptans vs placebo in Adolescents, outcome: 7.1 Pain‐free.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings 1. Should ibuprofen be used to treat children with migraine?
| Ibuprofen compared with placebo in children with migraine | |||||
| Patient or population: acute treatment of migraine in children Setting: ambulatory Intervention: ibuprofen Comparison: placebo | |||||
| Outcomes | Anticipated absolute effectsa (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Response with placebo | Response with Ibuprofen | ||||
| Pain freedom at 2 h | Study population | RR 1.87 (1.15 to 3.04) | 125 (2 RCTs) | ⨁⨁◯◯b,c Low | |
| 267 per 1000 | 499 per 1000 (307 to 811) | ||||
| Adverse events | 100 per 1000 | 0 per 1000 (− 13 to 13) |
RD 0.00 (− 0.13 to 0.13) |
80 (1 RCT) |
|
|
GRADE Working Group grades of evidence
High quality: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. CI: confidence interval; RR: risk ratio; RD: risk difference | |||||
aThe response in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). bIn the two studies, there were no serious risks of bias, inconsistency, indirectness, or publication bias. We downgraded quality of evidence by two levels due to very serious imprecision (small sample size, few events, and wide confidence interval).
cHigh (⨁⨁⨁⨁) = further research is very unlikely to change our confidence in the estimate of effect; Moderate (⨁⨁⨁◯) = further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; Low (⨁⨁◯◯) = further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; Very Low (⨁◯◯◯) = any estimate of effect is very uncertain.
Summary of findings 2. Should triptans be used to treat children with migraine?
| Triptans compared with placebo in children with migraine | ||||||
| Patient or population: acute treatment of migraine in children Setting: ambulatory Intervention: triptans Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effectsa (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Response with placebo | Response with triptans | |||||
| Pain freedom at 2 h | Study population | RR 1.67 (1.06 to 2.62) | 345 (3 RCTs) | ⨁⨁⨁◯b,c MODERATE | Includes rizatriptan oral (1 study) and sumatriptan by nasal spray (2 studies) | |
| 276 per 1000 | 461 per 1000 (292 to 723) | |||||
| Adverse events | 176 per 1000 | 11 per 1000 (− 7 to 30) |
RD 0.06 (− 0.04 to 0.17) |
420 (3 RCTs) |
||
|
GRADE Working Group grades of evidence
High quality: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. CI: confidence interval; RR: risk ratio; RD: risk difference. | ||||||
aThe response in the intervention group (and its 95% confidence interval) is based on the assumed response in the comparison group and the relative effect of the intervention (and its 95% CI). bIn the three studies, there were no serious risks of bias, inconsistency, indirectness, or publication bias detected. Quality of evidence was downgraded by one level due to serious imprecision (small sample size, few events, and wide confidence interval).
cHigh (⨁⨁⨁⨁) = further research is very unlikely to change our confidence in the estimate of effect; Moderate (⨁⨁⨁◯) = further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; Low (⨁⨁◯◯) = further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; Very Low (⨁◯◯◯) = any estimate of effect is very uncertain.
Summary of findings 3. Should triptans be used to treat adolescents with migraine?
| Triptans compared with placebo in adolescents with migraine | ||||||
| Patient or population: acute treatment of migraine in adolescents Setting: ambulatory Intervention: Triptans Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effectsa (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Response with placebo | Response with Triptans | |||||
| Pain freedom at 2 h | Study population | RR 1.32 (1.19 to 1.47) | 6761 (21 RCTs) | ⨁⨁⨁◯b,c MODERATE | Includes almotriptan (1 study), eletriptan (1 study), naratriptan (1 study), rizatriptan (4 studies), sumatriptan (10 studies), and zolmitriptan (4 studies) | |
| 230 per 1000 | 303 per 1000 (273 to 338) | |||||
| Adverse events | 184 per 1000 | 24 per 1000 (15 to 33) |
RD 0.13 (0.08 to 0.18) |
7876 (21 RCTs) |
||
|
GRADE Working Group grades of evidence
High quality: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. CI: confidence interval; RR: risk ratio; RD: risk difference. | ||||||
aThe response in the intervention group (and its 95% confidence interval) is based on the assumed response in the comparison group and the relative effect of the intervention (and its 95% CI). bSerious inconsistency was observed in the effect estimates. All of the triptans with only 1 study were not statistically superior to placebo (i.e. almotriptan, eletriptan, naratriptan) in producing pain freedom while the three triptans with 2 or more studies (i.e. rizatriptan, sumatriptan, and zolmitriptan) were statistically significant with a higher magnitude of effect. In the subgroup analysis of the individual triptan groups through, the subgroup differences were not statistically significant (P = 0.45).
cHigh (⨁⨁⨁⨁) = further research is very unlikely to change our confidence in the estimate of effect; Moderate (⨁⨁⨁◯) = further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; Low (⨁⨁◯◯) = further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; Very Low (⨁◯◯◯) = any estimate of effect is very uncertain.
Summary of findings 4. Should sumatriptan plus naproxen sodium be used to treat adolescents with migraine?
| Sumatriptan + naproxen sodium compared with placebo in adolescents with migraine | ||||||
| Patient or population: acute treatment of migraine in adolescents Setting: ambulatory Intervention: sumatriptan + naproxen sodium Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effectsa (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Response with placebo | Response with Sumatriptan + naproxen sodium | |||||
| Pain freedom at 2 h | Study population | RR 2.66 (1.57 to 4.51) | 485 (1 RCT) | ⨁⨁⨁◯b,c MODERATE | Doses including sumatriptan + naproxen 10 mg + 60 mg, 30 mg + 180 mg, and 85 mg + 500 mg were all well tolerated and demonstrated similar efficacy | |
| 99 per 1000 | 262 per 1000 (163 to 394) | |||||
| Adverse events | 83 per 1000 | 2 per 1000 (− 2 to 7) |
RD 0.03 (− 0.02 to 0.09) |
490 (1 RCT) |
||
|
GRADE Working Group grades of evidence
High quality: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. CI: confidence interval; RR: risk ratio; RD: risk difference. | ||||||
aThe response in the intervention group (and its 95% confidence interval) is based on the assumed response in the comparison group and the relative effect of the intervention (and its 95% CI). bThe confidence interval of the effect size is wide. The true effect may be substantially different than the estimated effect in producing pain freedom.
cHigh (⨁⨁⨁⨁) = further research is very unlikely to change our confidence in the estimate of effect; Moderate (⨁⨁⨁◯) = further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; Low (⨁⨁◯◯) = further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; Very Low (⨁◯◯◯) = any estimate of effect is very uncertain.
We describe the measures of effect for each of the interventions below. In addition, we present 'Summary of findings' tables for all comparisons for which there was more than one study.
Paracetamol versus placebo in children
In the one three‐way cross‐over study that evaluated paracetamol (Hämäläinen 1997a), the participant age ranged from 4 to 15.8 years (N = 88), but investigators did not report results for children and adolescents separately. However, the mean age of inclusion was 10.7 years, so we deemed the study to be predominantly in children. Paracetamol was not superior to placebo for the pain‐free outcome ( RR 1.40, 95% CI 0.75 to 2.58). There was no statistically significant difference in headache relief (defined as a reduction in pain by two grades on a five point scale), rescue medication, headache recurrence, or adverse events. The study did not report the presence of nausea or vomiting.
Ibuprofen versus placebo in children
Ibuprofen was superior to placebo in the pooled analysis of two studies (Figure 5) ‐ Hämäläinen 1997a was a three‐way cross‐over study with paracetamol, with a mean participant age of 10.7 years (N = 88), and Lewis 2002 was a parallel group study that included only 6 to 12 year‐olds with a mean age of 9 years (N = 84). For the pain‐free primary outcome, the RR was 1.87 (95% CI 1.15 to 3.04; Analysis 1.1) with a NNTB of 4. Ibuprofen was also superior to placebo for headache relief (Analysis 1.3) but not for rescue medication (Analysis 1.4) or headache recurrence (Analysis 1.5). There was no difference in the proportion of adverse events between groups (Analysis 1.2). Lewis 2002 did not report adverse events, and Hämäläinen 1997a did not report on the presence of nausea and vomiting.
5.

Forest plot of comparison: 2 Ibuprofen vs placebo in Children, outcome: 2.1 Pain‐free.
1.1. Analysis.

Comparison 1: Ibuprofen vs placebo in children, Outcome 1: Pain‐free
1.3. Analysis.

Comparison 1: Ibuprofen vs placebo in children, Outcome 3: Headache relief
1.4. Analysis.

Comparison 1: Ibuprofen vs placebo in children, Outcome 4: Rescue medication
1.5. Analysis.

Comparison 1: Ibuprofen vs placebo in children, Outcome 5: Headache recurrence
1.2. Analysis.

Comparison 1: Ibuprofen vs placebo in children, Outcome 2: Adverse events (any)
The quality of evidence for the pain‐free outcome was low, downgraded by two levels due to very serious imprecision (Table 1).
Ibuprofen versus placebo in adolescents
One small three‐way cross‐over study examined ibuprofen, zolmitriptan, and placebo (Evers 2006). While it included both children and adolescents, the mean participant age was 13.9 years (N = 29), so we considered the study to be predominantly in adolescents. Headache relief at two hours was the primary outcome measure reported, but investigators also reported pain freedom at two hours. The pain‐free outcome was not statistically significant (RR 7.00, 95% CI 0.99 to 49.69), but ibuprofen was statistically superior to placebo for headache relief (RR 2.50, 95% CI 1.02 to 6.10). There were no significant differences in other secondary outcome measures, including use of rescue medication, headache recurrence, presence of nausea, or presence of vomiting. There was no significant difference in adverse events observed.
Triptans versus placebo in children
Three studies examined two triptan medications in children under 12 years of age: rizatriptan (Ho 2012, N = 200) and sumatriptan (Hämäläinen 2002, N = 59; Ueberall 1999, N = 14). Triptans as a class of medication were superior to placebo in children for the primary outcome measure of pain freedom (RR 1.67, 95% CI 1.06 to 2.62; Analysis 2.1) with a NNTB of 13. There were no statistically significant differences in the effect size between rizatriptan and sumatriptan subgroups. Overall, we did not observe any statistically significant difference in the secondary outcomes of headache relief (Analysis 2.3), rescue medication (Analysis 2.4), headache recurrence (Analysis 2.5), presence of nausea (Analysis 2.6), or presence of vomiting (Analysis 2.7). There was no statistically significant difference in the proportion of adverse events observed in the triptan versus placebo groups (Analysis 2.2).
2.1. Analysis.

Comparison 2: Triptans vs placebo in children, Outcome 1: Pain‐free
2.3. Analysis.

Comparison 2: Triptans vs placebo in children, Outcome 3: Headache relief
2.4. Analysis.

Comparison 2: Triptans vs placebo in children, Outcome 4: Rescue medication
2.5. Analysis.

Comparison 2: Triptans vs placebo in children, Outcome 5: Headache recurrence
2.6. Analysis.

Comparison 2: Triptans vs placebo in children, Outcome 6: Presence of nausea
2.7. Analysis.

Comparison 2: Triptans vs placebo in children, Outcome 7: Presence of vomiting
2.2. Analysis.

Comparison 2: Triptans vs placebo in children, Outcome 2: Adverse events (any)
The quality of evidence for the pain‐free outcome was moderate, downgraded by one level due to serious imprecision (Table 2).
Triptans versus placebo in adolescents
Triptans as a class of medications were superior to placebo in adolescents for the acute treatment of migraine (Figure 6). Overall the pain‐free RR was 1.32 (95% CI 1.19 to 1.47; Analysis 3.1) with a NNTB of 6 and low (I2 = 26%), non‐significant heterogeneity (P = 0.13) between studies. Triptans for which there were two or more studies were statistically superior to placebo as a subgroup (Figure 6), but subgroup differences in effect size were not statistically significant. The individual triptans included were almotriptan (Linder 2008, N = 714), eletriptan (Winner 2007, N = 274), naratriptan (Rothner 1997, N = 300), rizatriptan (Ahonen 2006, N = 96; Winner 2002, N = 296; Ho 2012, N = 570; Visser 2004a, N = 476), sumatriptan (Ahonen 2004, N = 83; Callenbach 2007, N = 46; Fujita 2014, N = 144; Hämäläinen 1997b, N = 23; Rothner 1999a, N = 273; Rothner 1999b, N = 92; Rothner 1999c, N = 102; Winner 1997, N = 298; Winner 2000, N = 507; Winner 2006, N = 731), and zolmitriptan (Evers 2006, N = 29; Lewis 2007, N = 171; NCT01211145, N = 584; Rothner 2006, N = 696). There was, however, an increased risk of minor (non‐serious) adverse events in the triptan group when compared with placebo, with an RD of 0.13 (95% CI 0.08 to 0.18; Analysis 3.2) and NNTH of 8. The secondary efficacy outcomes that favoured triptans were headache relief (RR 1.14, 95% CI 1.04 to 1.24; Analysis 3.3), a reduction in the use of rescue medication (RR 0.79, 95% CI 0.72 to 0.87; Analysis 3.4), and reduced risk of headache recurrence (RR 0.79, 95% CI 0.68 to 0.93; Analysis 3.5). There were no statistically significant differences in the presence of nausea (Analysis 3.6) or vomiting (Analysis 3.7).
6.

Forest plot of comparison: 7 Triptans vs placebo in Adolescents, outcome: 7.1 Pain‐free.
3.1. Analysis.

Comparison 3: Triptans vs placebo in adolescents, Outcome 1: Pain‐free
3.2. Analysis.

Comparison 3: Triptans vs placebo in adolescents, Outcome 2: Adverse events (any)
3.3. Analysis.

Comparison 3: Triptans vs placebo in adolescents, Outcome 3: Headache relief
3.4. Analysis.

Comparison 3: Triptans vs placebo in adolescents, Outcome 4: Rescue medication
3.5. Analysis.

Comparison 3: Triptans vs placebo in adolescents, Outcome 5: Headache recurrence
3.6. Analysis.

Comparison 3: Triptans vs placebo in adolescents, Outcome 6: Presence of nausea
3.7. Analysis.

Comparison 3: Triptans vs placebo in adolescents, Outcome 7: Presence of vomiting
The quality of evidence for the pain‐free outcome was moderate, downgraded by one level due to serious inconsistency (Table 3).
Sumatriptan plus naproxen sodium versus placebo in adolescents
We included one study of sumatriptan plus naproxen sodium versus placebo. The study included adolescents with a mean age of 14.7 years and randomized a total of 683 participants. The doses of sumatriptan + naproxen sodium, respectively, were 10 mg + 60 mg (N = 96), 30 mg + 180 mg (N = 97), and 85 mg + 500 mg (N = 152). The primary outcome reported was pain freedom at two hours, with data adjusted for age and baseline severity. The adjusted pain‐free rates at two hours were higher with sumatriptan + naproxen sodium 10 mg + 60 mg (29%; adjusted P = 0.003), 30 mg + 180 mg (27%; adjusted P = 0.003), and 85 mg + 500 mg (24%; adjusted P = 0.003) versus placebo (10%). Post hoc analyses showed no differences among the 3 doses or an age‐by‐treatment interaction. Calculating the RR for pain relief at 2 hours, sumatriptan + naproxen sodium was superior to placebo with an RR of 3.25 (95% CI 1.78 to 5.94) and NNTB of 6. The use of rescue medication was also significantly reduced with the combination medication when compared with placebo (RR 0.46, 95% CI 0.32 to 0.64), but investigators did not report headache relief. There was no statistically significant increase in adverse events and no difference in the presence of nausea. Headache recurrence and presence of vomiting were also not reported.
The quality of evidence for the pain‐free outcome was moderate, downgraded by one level due to the width of the confidence interval of the effect size (Table 4).
Dihydroergotamine (DHE) versus placebo in children
One small cross‐over study of dihydroergotamine versus placebo included children and adolescents aged 5 to 15 years (Hämäläinen 1997c, N = 13). With a mean participant age of 10.3 years, we considered the study to be predominantly in children. DHE demonstrated no significant difference in the pain‐free outcome or any of the secondary outcomes, including headache relief or the use of rescue medications. There was no statistically significant increase in the proportion of adverse events between DHE and placebo. Authors did not report headache recurrence, presence of nausea, or the presence of vomiting.
Subgroup analysis for other sources of heterogeneity
We assessed potentially important sources of clinical heterogeneity using the pain‐free outcome of triptan studies in adolescents. Tests for subgroup differences showed that effect size estimates for intranasal studies of sumatriptan and zolmitriptan were significantly higher than oral triptan studies of almotriptan, eletriptan, naratriptan, rizatriptan, sumatriptan, and zolmitriptan (P = 0.02, I2 = 81.5%, Analysis 4.1). We observed a statistically significant difference in the comparison of oral versus intranasal zolmitriptan studies, where intranasal delivery was associated with a significantly higher effect size for the pain‐free outcome (P = 0.04)=, I2 = 77.1%; Analysis 4.3). However, the difference was not statistically significant when comparing oral versus intranasal sumatriptan studies (Analysis 4.2). The permitted concomitant use of migraine preventive medications in the triptan trials among adolescents was not associated with any significant difference in the effect size (Analysis 4.4). We did not observe any statistically significant differences in the overall effect size estimates between the triptan versus placebo studies that included only children, those where children and adolescents were mixed and not reported separately, and those studies that examined adolescents exclusively (P = 0.42, I2 = 0%; Analysis 5.1).
4.1. Analysis.

Comparison 4: Triptans vs placebo in adolescents, subgroup analysis, Outcome 1: Pain‐free by route (oral or intranasal)
4.3. Analysis.

Comparison 4: Triptans vs placebo in adolescents, subgroup analysis, Outcome 3: Zolmitriptan vs placebo by route (oral or intranasal)
4.2. Analysis.

Comparison 4: Triptans vs placebo in adolescents, subgroup analysis, Outcome 2: Sumatriptan vs placebo by route (oral or intranasal)
4.4. Analysis.

Comparison 4: Triptans vs placebo in adolescents, subgroup analysis, Outcome 4: Pain‐free by preventive medication
5.1. Analysis.

Comparison 5: Triptans vs placebo by age, subgroup analysis, Outcome 1: Age group
Sensitivity analysis of other study characteristics
The overall heterogeneity was low for the triptan versus placebo studies in adolescents for the pain‐free outcome (Tau2 = 0.01; Chi2 = 27.15, degrees of freedom (df) = 20 (P = 0.13); I2 = 26%). We performed a sensitivity analysis to explore the effects of sponsorship; risk of bias in allocation concealment; study design (cross‐over versus parallel group); type of study report (journal article versus clinical trial registry and abstract); and small sample size (< 50). The cross‐over study design was associated with significantly higher effect size estimates for the pain‐free outcome (P = 0.004, I2 = 88.2%; Analysis 6.1). The effect estimate for the triptan versus placebo studies in adolescents was similar in direction, magnitude, and significance (i.e. RR 1.25, 95% CI 1.12 to 1.39) when we removed studies of cross‐over design. Similarly studies with a sample size of less than 50 had a significantly higher estimates of treatment effect (P = 0.03, I2 = 79.4%; Analysis 6.5). The overall effect estimate without studies having small sample sizes was similar to the reported effect estimate (RR 1.31, 95% CI 1.18 to 1.46). There were no significant subgroup difference for allocation concealment (Analysis 6.2), source of funding (Analysis 6.3), or type of study report (Analysis 6.4).
6.1. Analysis.

Comparison 6: Triptans vs placebo in adolescents, sensitivity analysis, Outcome 1: Study design
6.5. Analysis.

Comparison 6: Triptans vs placebo in adolescents, sensitivity analysis, Outcome 5: Sample size
6.2. Analysis.

Comparison 6: Triptans vs placebo in adolescents, sensitivity analysis, Outcome 2: Allocation concealment
6.3. Analysis.

Comparison 6: Triptans vs placebo in adolescents, sensitivity analysis, Outcome 3: Source of funding
6.4. Analysis.

Comparison 6: Triptans vs placebo in adolescents, sensitivity analysis, Outcome 4: Reported in a journal
Discussion
Summary of main results
In total, we identified 27 moderate quality studies for inclusion. Most were at a low or unclear risk of bias and of varying size (a range of 13 to 888 participants in total). The mean age of inclusion was 12.9 years with a range of means between 8.2 and 14.7 years.
Paracetamol
There is insufficient evidence in favor of paracetamol for the acute treatment of migraine in children or adolescents. We only identified one small cross‐over study, predominantly in children, where oral paracetamol was not superior to placebo or ibuprofen.
Ibuprofen
Ibuprofen was more effective than placebo in producing pain freedom in two small studies involving children. In one small cross‐over study in adolescents, ibuprofen was not superior to placebo for pain freedom, but it was for headache relief (Evers 2006). While ibuprofen as a non‐steroidal anti‐inflammatory drug (NSAID) may have some advantage in the treatment of a migraine attack, considering the presence of neurogenic inflammation (Levy 2008), it was not superior to zolmitriptan or paracetamol in two three‐way cross‐over studies (Evers 2006; Hämäläinen 1997a). Ibuprofen was not associated with an increase in adverse events overall.
Triptans
Triptans as a class of medication were more effective than placebo in producing pain freedom in 3 studies involving children and 21 studies involving adolescents. We did not observe any significant differences in the effect sizes between the subgroups of individual triptans, including almotriptan, eletriptan, naratriptan, rizatriptan, sumatriptan, or zolmitriptan. While there was some evidence to suggest that intranasal preparations of sumatriptan and zolmitriptan produce higher effect sizes than oral preparations of all the triptans listed above, the evidence was inconsistent. Evidence for the efficacy of the triptans was variable, as measured through the secondary outcomes of headache relief, use of rescue medication, headache recurrence, presence of nausea, and presence of vomiting, but in general it favored the triptans. The efficacy, however, is counterbalanced with an increased risk of minor (non‐serious) adverse events overall. However, there were no serious adverse events reported. Commonly reported adverse events included fatigue, dizziness, asthenia, dry mouth, and nausea or vomiting with oral preparations, and taste disturbance, nasal symptoms, and nausea with intranasal preparations. The combination of sumatriptan with naproxen sodium was also more effective than placebo in producing pain freedom in one adolescent study. We did not observe any significant differences in the effect sizes between studies that included children versus adolescents. The only significant study characteristic that was associated with a higher effect size was the use of a cross‐over design versus parallel group design.
Dihydroergotamine
There is insufficient evidence for oral dihydroergotamine in the treatment of migraine in children or adolescents. We only identified one small cross‐over study, which found that oral dihydroergotamine was not superior to placebo.
Overall completeness and applicability of evidence
Of the 27 randomized controlled trials we identified on migraine symptom relieving medications used in the outpatient setting for children and adolescents, 24 belonged to the triptan class, including almotriptan, eletriptan, naratriptan, rizatriptan, sumatriptan, and zolmitriptan. Pharmaceutical companies sponsored most of the triptan studies (N = 19). We only identified three studies of the most frequently used pain medications (paracetamol and ibuprofen) and none examining other non‐steroidal anti‐inflammatory drugs (NSAIDs) or early treatment (Goadsby 2008; Suthisisang 2010). We only identified one study on a combination medication (sumatriptan plus naproxen sodium).
Quality of the evidence
We judged the quality of evidence for the effect of triptans on pain relief to be moderate, having downgraded it due to serious inconsistency in adolescents and imprecision in children (small sample size, few events, and wide confidence intervals. For ibuprofen, we downgraded the quality of the evidence by two levels to low due to very serious imprecision (small sample size, few events, and wide confidence intervals). It is likely that future research will help to tighten the confidence intervals around the effect size of the above medications. More evidence is needed to assess the effect of paracetamol in children and adolescents. Although we identified heterogeneity between the results in the triptan studies, we believe that further research is unlikely to change the direction of the effect.
Potential biases in the review process
The reviewers searched the indexed and gray literature extensively to identify all published and unpublished studies of medications used in the treatment of migraine in children and adolescents. Pharmaceutical company clinical trial registries were available for sumatriptan, sumatriptan + naproxen sodium, rizatriptan, and zolmitriptan studies, and we identified some negative trials in these sources. The absence of trial registries for the other medications may bias the identification of other negative trials. The funnel plot examination suggested some potential for publication bias, but the Egger statistical tests for bias were not significant.
Agreements and disagreements with other studies or reviews
A number of similar systematic reviews have been published. The review by Major 2003 of triptan studies in children and adolescents aged 6 to 18 years concluded that intranasal sumatriptan was effective, while other oral triptans were not. The American Academy of Neurology Quality Standards Subcommittee and the Practice Committee of the Child Neurology Society have published a practice parameter on the pharmacological treatment of migraine (Lewis 2004). Our conclusions are similar with regard to the evidence for efficacy of ibuprofen in children but differ with regard to the efficacy of paracetamol. The practice parameter also concluded that sumatriptan nasal spray was effective for the acute treatment of migraine in adolescents. Damen 2005 evaluated all randomized controlled trials for the acute treatment of migraine in children and adolescents less than 18 years of age but identified a smaller number of trials (10 studies). The authors performed a meta‐analysis, and concluded there was evidence for the use of ibuprofen, paracetamol, and intranasal sumatriptan. Silver 2008 also included all medications for the acute treatment of migraine in their search, but identified only 11 studies in children and adolescents under 18 years of age. The authors concluded that there was evidence only for the use of ibuprofen and sumatriptan and did not differentiate between oral and intranasal sumatriptan preparations. A meta‐analysis and qualitative review of ibuprofen and paracetamol in adults, adolescents, and children concluded that ibuprofen was at least as efficacious as paracetamol (Pierce 2010). Eiland 2010 recommended the triptan class as an acute treatment option for children and adolescents with migraines, also concluding that there was evidence to recommend sumatriptan and zolmitriptan nasal sprays as well as rizatriptan or almotriptan tablets over the other triptans. Vollono 2011 examined 11 studies and concluded that triptans are an important option in the symptomatic treatment of childhood and adolescent migraine. Individually, they found that zolmitriptan and rizatriptan were superior to placebo in most studies, and almotriptan was well tolerated. Toldo 2012 recommended paracetamol and ibuprofen as first line treatments for migraine in children and adolescents. The triptans were deemed safe, and the authors concluded that sumatriptan nasal spray was more effective than placebo and that zolmitriptan nasal spray and rizatriptan tablets were likely effective. Wöber‐Bingöl 2013 examined 14 studies on triptans in adolescents and 6 studies in children and concluded that evidence for the acute pharmacological treatment of migraine in children was poor, but evidence for adolescents was better, albeit still limited. The authors outlined that sumatriptan nasal spray and zolmitriptan nasal spray were approved for adolescents in Europe; while in the United States almotriptan can be used for adolescents and rizatriptan is approved for patients aged 6 to 17 years. The combination of sumatriptan and naproxen sodium in adolescents has also since been approved for use in adolescents in the United States. Bonfert 2013 included 33 studies examining acute and preventive treatment for migraine and tension‐type headaches. The reviewers did not conduct a meta‐analysis but reviewed the individual studies, concluding that ibuprofen and paracetamol should be considered first and second line therapies for the acute treatment of migraine in children and adolescents, with almotriptan and rizatriptan as suitable third‐line agents.
Finally, a recent review identified only seven studies in adolescents aged 12 to 17 years and concluded that enrichment designs significantly reduced placebo response (Sun 2013). Our review includes a higher number of studies (N = 27), including negative studies published only in pharmaceutical‐industry sponsored trial registries. We draw similar conclusions with regard to the benefit and safety of ibuprofen in treating children and adolescents with migraine. As with previous reviews, the low cost, broad availability, and safety may make ibuprofen a preferred first choice. We differ however in our conclusion that there is insufficient evidence to recommend paracetamol. In general our conclusions are similar with regard to the triptans as a class of medication being effective in the treatment of children and adolescents with migraine. We differ in that we found insufficient evidence to recommend one triptan over the others in our meta‐analysis and sub‐group analyses. While rizatriptan, sumatriptan, and zolmitriptan were statistically superior to placebo as subgroups, the overall test for subgroup heterogeneity was not significant. In our meta‐analysis, we combined the studies of triptan medications irrespective of route of delivery and dosage.
Authors' conclusions
Implications for practice.
We found low quality evidence from two small studies that ibuprofen appears to improve pain relief in children with migraine. We have only limited information on adverse events in the trials included in this review. There is insufficient evidence in favor of paracetamol. We did not identify evidence regarding early treatment, the use of other NSAIDs, or the combination of these analgesics with other medications (e.g. metoclopramide, caffeine) in children or adolescents.
For children and adolescents with migraine, triptans appear to be effective in the acute treatment of migraine. Sumatriptan plus naproxen sodium is effective in adolescents, but we did not find any studies in children. Triptans are generally safe but carry an increased risk of minor (non‐serious) adverse events.
For clinicians, the choice of triptan medication may be guided by factors such as patient preference, route of delivery, or palatability. A parent who responds well to one of the medications may be more likely to request that medication for their child. More than half of the triptan studies in children and adolescents were of sumatriptan, and this medication serves as the reference drug in this class. The choice of triptan may also be based on local availability, drug cost reimbursement, and regulatory approval. We found inconsistent evidence in our subgroup analysis of the intranasal versus oral triptan preparations, but patients may prefer one route over the other (e.g. intranasal if significant vomiting or oral if patient has chronic rhinitis).
For policymakers and funders, the triptan class of medications, as well as sumatriptan in combination with naproxen sodium, are suitable options for children and adolescents with migraine. One or more of these medications should be made available in situations where ibuprofen has failed to provide pain freedom or headache relief.
Implications for research.
General
Future studies should separate the childhood and adolescent age groups to enable separate meta‐analyses of these groups. More studies of simple analgesics commonly used in the treatment of migraine like paracetamol and ibuprofen, other NSAIDs, or head to head comparisons are warranted.
Design
Studies employing a cross‐over design were associated with significantly higher effect size estimates. Similar findings have been reported previously (Lathyris 2007). This may have been due to inadequate blinding in cross‐over studies or higher effect estimates due to the smaller sample size. Guidelines for controlled trials of drugs in migraine advocate the use of either cross‐over or parallel design (Tfelt‐Hansen 2012).
Measurement (endpoints)
Pain freedom as an outcome measure demonstrated low heterogeneity between studies and may be most suitable as a primary outcome measure. Data on other outcome measures (e.g. nausea, headache relief, etc.) should also be collected, as described in the guidelines for controlled trials of drugs in migraine (Tfelt‐Hansen 2012).
What's new
| Date | Event | Description |
|---|---|---|
| 29 October 2020 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 2, 2005 Review first published: Issue 4, 2016
| Date | Event | Description |
|---|---|---|
| 17 October 2018 | Review declared as stable | See Published notes. |
| 1 September 2010 | Feedback has been incorporated | Incorporated feedback from Editorial review and updated search. |
| 15 May 2008 | Amended | Converted to new review format. |
Notes
This systematic review has not previously been published in full form. The data have been presented at the American Headache Society Annual Scientific meeting.
Assessed for updating in 2018
A full search was performed in February 2018 and after screening the results the authors did not identify any potentially relevant studies. At October 2018, this review has been stabilised following discussion with the authors and editors. New treatments for migraine are anticipated, and we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Assessed for updating in 2020
At October 2020 we are not aware of any potentially relevant studies likely to change the conclusions, although this is an active area of research and new studies are expected in the next two to three years. Following discussion with the authors and editors, this review has now been stabilised and will be reassessed for updating in two years. If appropriate we will update the review sooner if new evidence likely to change the conclusions is published, or if standards change substantially which necessitates major revisions.
Acknowledgements
Dr. Lawrence Richer wishes to thank Dr. Brian Rowe for mentoring his Master of Science in Clinical Epidemiology training and thesis preparation.
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane PaPaS Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. Complete Search Strategy
LITERATURE SEARCH—Drugs for the acute treatment of migraine in children and adolescents
| Database | 2008 | 2012 | 2013 | 2014 | 2016 | Total | ||||||
| Number retrieved | After duplicates removed | Number retrieved | After duplicates removed | Number retrieved | After duplicates removed | Number retrieved | After duplicates removed | Number retrieved | After duplicates removed | Number retrieved | After duplicates removed | |
| MEDLINE | 2844 | 2742 | 639 | 616 | 90 | 75 | — | — | 326 | 231 | 3899 | 3664 |
| MEDLINE In‐Process | 29 | 29 | 29 | 29 | 16 | 14 | — | — | — | — | 74 | 72 |
| CCRT | 153 | 126 | 153 | 126 | 10 | 2 | — | — | — | — | 316 | 254 |
| CDSR DARE | 664 | 650 | 664 | 650 | 324 | 169 | — | — | — | — | 1652 | 1469 |
| IPA | 109 | 27 | 40 | 10 | 7 | 4 | — | — | — | — | 156 | 41 |
| PsycINFO | 255 | 147 | 147 | 85 | 5 | 3 | — | — | — | — | 407 | 235 |
| EMBASE | 6357 | 5548 | 2884 | 2517 | 508 | 412 | 794 | 787 | 626 | 516 | 11,169 | 9780 |
| CINAHL | 712 | 190 | 263 | 70 | 31 | 21 | — | — | — | — | 1006 | 281 |
| Total | 11,123 | 9459 | 4819 | 4103 | 991 | 700 | 794 | 787 | 952 | 747 | 18,679 | 15,796 |
Searcher: Robin Featherstone
Requestor: Lawrence Richer
Date Submitted: 3 February 2016
Last update: 19 December 2014
Files submitted:
1. Richer‐AcuteMigraine‐Update_Feb2016.enlx
2. Richer‐AcuteMigraine‐Update_Feb2016.xlsx
Search Summary:
| Database | Date Searched | Number Retrieved | After Duplicate Removala |
| EMBASE | 3 February 2016 | 626 | 516 |
| MEDLINE | 3 February 2016 | 326 | 231 |
| Total | 952 | 747 |
aNote: Removed any records retrieved by previous updates.
Database: EMBASE via Ovid 1996 to 2016 Week 05
Search Title: Richer_Acute_Migraine_2016Update
Strategy:
| Migraine‐related terms: 1. exp Headache Disorders/ 2. vascular headache/ 3. headache/ 4. (migraine$ or headache$ or head‐ache$ or cephalgia or cephalalgia).ti,ab. 5. or/1‐4 (180,453) Pharmaceutical related terms: 6. exp Drug Therapy/ 7. (drug adj3 (therap$ or treatment?)).mp. 8. ((anti‐migrain$ or antimigrain$) adj3 (therap$ or treatment?)).mp. 9. (ad or ae or dt or to).fs. 10. exp Treatment Outcome/ 11. exp Analgesics/ 12. "nonsteroidal anti‐inflammatory agent?".mp. 13. "non‐steroidal anti‐inflammatory agent?".mp. 14. NSAID?.mp. 15. ibuprofen.mp. 16. fenoprofen.mp. 17. flurbiprofen.mp. 18. ketoprofen.mp. 19. ketorolac.mp. 20. diclofenac.mp. 21. etodolac.mp. 22. sulindac.mp. 23. diflunisal.mp. 24. naproxen.mp. 25. oxaprozin.mp. 26. tiaprofenic acid.mp. 27. mefenamic acid.mp. 28. indomethacin.mp. 29. tolmetin.mp. 30. celecoxib.mp. 31. meloxicam.mp. 32. piroxicam.mp. 33. tenoxicam.mp. 34. floctafenin$.mp. 35. nabumeton$.mp. 36. acetaminophen.mp. 37. paracetamol.mp. 38. ergot$ alkaloid?.mp. 39. ergotamin$.mp. 40. dihydroergotoxin$.mp. 41. dihydroergotamin$.mp. 42. DHE.mp. 43. ergoloid mesylates.mp. 44. methysergide.mp. 45. ziconotide.mp. 46. opioid$.mp. 47. opiate$.mp. 48. opium.mp. 49. meperidine.mp. 50. alfentan#l.mp. 51. fentan#l.mp. 52. rem#fentan#l.mp. 53. sufentan#l.mp. 54. levomethadyl.mp. 55. butorphanol.mp. 56. codein?.mp. 57. morphine.mp. 58. pentazocin$.mp. 59. (propoxyphen$ or dextro?propoxyphen$).mp. 60. nalbuphin$.mp. 61. hydromorphon$.mp. 62. oxycodon$.mp. 63. oxymorphon$.mp. 64. methadon$.mp. 65. butalbital.mp. 66. aspirin.mp. 67. acetylsalicylic acid.mp. 68. caffeine.mp. 69. "combination analgesic?".tw. 70. APAP.tw. 71. dichloralphenazone.mp. 72. isomethepten$.mp. 73. corticosteroid$.mp. 74. hydrocortisone.mp. 75. prednisolone.mp. 76. methylprednisolone.mp. 77. dexamethasone.mp. 78. tryptamin$.mp. 79. triptan?.mp. 80. sumatriptan.mp. 81. naratriptan.mp. 82. rizatriptan.mp. 83. zolmitriptan.mp. 84. almotriptan.mp. 85. eletriptan.mp. 86. frovatriptan.mp. 87. serotonin agonist?.mp. 88. ((5‐hydroxytryptamine or 5‐HT) adj2 agonist?).mp. 89. (antiemetic? or anti‐emetic?).mp. 90. (antinauseant? or anti‐nauseant?).mp. 91. chlorpromazine.mp. 92. prochlorperazine.mp. 93. perphenazine.mp. 94. trifluoperazine.mp. 95. (met#clopr#mide or metochlopramide).mp. 96. scopolamin$.mp. 97. dimenhydrinate.mp. 98. dronabinol.mp. 99. nabilon$.mp. 100. thiethylperazine.mp. 101. trimethobenzamide.mp. 102. ondansetron.mp. 103. granisetron.mp. 104. dolasetron.mp. 105. diphenhydramine.mp. 106. hydroxyzine.mp. 107. promethazine.mp. 108. Valproic Acid.mp. 109. valproate.mp. 110. divalproex sodium.mp. 111. Clonidine.mp. 112. fluid bolus.mp. 113. normal saline.mp. 114. magnesium.mp. 115. lidocaine.mp. 116. Botulinum Toxin Type A/ 117. botulinium toxin.mp. 118. botox.mp. 119. oxygen.mp. 120. placebo$.mp. 121. or/6‐120 [combination of all pharmaceutical treatments for migraine] RCT filter: 122. random*.tw. 123. placebo*.mp. 124. double‐blind*.tw. 125. or/122‐124 [RCT filter from J Med Libr Assoc 2006] Child related terms: 126. adolescent/ 127. child/ 128. newborn/ 129. exp Pediatrics/ 130. infant$.mp. 131. infancy.mp. 132. newborn$.mp. 133. baby.mp. 134. babies.mp. 135. neonat$.mp. 136. preterm$.mp. 137. prematur$.mp. 138. postmatur$.mp. 139. child$.mp. 140. kid.mp. 141. kids.mp. 142. toddler$.mp. 143. adolescen$.mp. 144. teen$.mp. 145. juvenile$.mp. 146. boy$.mp. 147. girl.mp. 148. girls.mp. 149. minor$.mp. 150. pubert$.mp. 151. pubescen$.mp. 152. pediatric$.mp. 153. paediatric$.mp. 154. peadiatric$.mp. 155. or/126‐154 [child filter as per original search] 156. and/5,121,125,155 [combination of migraine + drugs + RCT + child] 157. limit 156 to em=201450‐201605 (639) 158. remove duplicates from 157 (626) |
Database: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) 1946 to Present
Search Title: Richer_Acute_Migraine_2016Update_1
Strategy:
| 1. exp Headache Disorders/ 2. vascular headaches/ 3. headache/ 4. (migraine$ or headache$ or head‐ache$ or cephalgia or cephalalgia).ti,ab. 5. or/1‐4 6. exp Drug Therapy/ 7. (ad or ae or dt or to).fs. 8. exp Treatment Outcome/ 9. exp Analgesics/ 10. "nonsteroidal anti‐inflammatory agent?".mp. 11. "non‐steroidal anti‐inflammatory agent?".mp. 12. NSAID?.mp. 13. ibuprofen.mp. 14. fenoprofen.mp. 15. flurbiprofen.mp. 16. ketoprofen.mp. 17. ketorolac.mp. 18. diclofenac.mp. 19. etodolac.mp. 20. sulindac.mp. 21. naproxen.mp. 22. tolmetin.mp. 23. oxaprozin.mp. 24. tenoxicam.mp. 25. tiaprofenic acid.mp. 26. mefenamic acid.mp. 27. ((acetylsalicylic adj1 acid) or aspirin).mp. 28. piroxicam.mp. 29. celecoxib.mp. 30. meloxicam.mp. 31. indomethacin.mp. 32. floctafenin$.mp. 33. nabumeton$.mp. 34. acetaminophen.mp. 35. paracetamol.mp. 36. ergotamin$.mp. 37. dihydroergotamin$.mp. 38. DHE.mp. 39. opioid$.mp. 40. opium.mp. 41. methadon$.mp. 42. meperidine.mp. 43. butorphanol.mp. 44. hydromorphon$.mp. 45. morphin$.mp. 46. codein?.mp. 47. butalbital.mp. 48. pentazocine.mp. 49. propoxyphene.mp. 50. nalbuphine.mp. 51. oxycodon$.mp. 52. ocymorphon$.mp. 53. alfentanil.mp. 54. fentanyl.mp. 55. sufentanil.mp. 56. caffeine.mp. 57. "combination analgesic?".tw. 58. tryptamines.mp. 59. triptan?.mp. 60. sumatriptan.mp. 61. naratriptan.mp. 62. rizatriptan.mp. 63. zolmitriptan.mp. 64. almotriptan.mp. 65. eletriptan.mp. 66. frovatriptan.mp. 67. serotonin agonist?.mp. 68. ((5‐hydroxytriptamine or 5‐HT) adj2 agonist?).mp. 69. (antiemetic? or anti‐emetic?).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 70. (antinauseant? or anti‐nauseant?).mp. 71. thiethylperazin$.mp. 72. trimethobenzamid$.mp. 73. scopolamin$.mp. 74. chlorpromazine.mp. 75. prochlorperazine.mp. 76. promethazin$.mp. 77. perphenazin$.mp. 78. trifluoperazin$.mp. 79. met#clopr#mide.mp. 80. ondansetron.mp. 81. granisetron.mp. 82. dolasetron.mp. 83. diphenhydramine.mp. 84. hydroxyzine.mp. 85. dimenhydrinate.mp. 86. dronabinol.mp. 87. nabilone.mp. 88. fluid bolus.mp. 89. normal saline.mp. 90. magnesium.mp. 91. lidocaine.mp. 92. corticosteroid$.mp. 93. prednisolone.mp. 94. solumedrol.mp. 95. dexamethason$.mp. 96. hydrocortisol.mp. 97. exp methylprednisolone/ 98. Botulinum Toxin Type A/ 99. botulinium toxin.mp. 100. botox.mp. 101. oxygen.mp. 102. clonidine.mp. 103. diflunisal.mp. 104. (levomethadyl or levo‐methadyl).mp. 105. remifentanil.mp. 106. ziconotid$.mp. 107. placebo$.mp. 108. (antimigrain$ or anti‐migrain$).mp. 109. divalproex.mp. 110. methysergid$.mp. 111. (ergoloid adj1 mesylate$).mp. 112. (abortive adj3 (therap$ or treatment$)).mp. 113. or/6‐112 114. and/5,113 115. clinical trial.pt. 116. randomized controlled trial.pt. 117. randomi?ed.ti,ab. 118. placebo.ti,ab. 119. dt.fs. 120. randomly.ti,ab. 121. trial.ti,ab. 122. groups.ti,ab. 123. or/115‐122 124. animals/ 125. humans/ 126. 124 not (124 and 125) 127. 123 not 126 128. exp Infant/ 129. exp Child/ 130. Adolescent/ 131. Minors/ 132. exp Puberty/ 133. exp Pediatrics/ 134. infant$.mp. 135. infancy.mp. 136. newborn$.mp. 137. baby.mp. 138. babies.mp. 139. neonat$.mp. 140. preterm$.mp. 141. prematur$.mp. 142. postmatur$.mp. 143. child$.mp. 144. kid.mp. 145. kids.mp. 146. toddler$.mp. 147. adolescen$.mp. 148. teen$.mp. 149. boy$.mp. 150. girl.mp. 151. minor$.mp. 152. pubert$.mp. 153. pubescen$.mp. 154. prepubescen$.mp. 155. pediatric$.mp. 156. paediatric$.mp. 157. peadiatric$.mp. 158. or/128‐156 159. and/114,127,158 160. limit 159 to ed=20141219‐20161231 161. remove duplicates from 160 |
‐
Searcher: Robin Featherstone (original strategy Andrea Milne)
Requestor: Lawrence Richer
Date Requested: 3 December 2014
Date Submitted: 19 December 2014
NOTE: Last update: April 2013
Search Summary:
| Database | Date Searched | Number Retrieved | After Duplicates Removed |
| EMBASE | 19 December 2014 | 794 | 787 |
Database: EMBASE via Ovid 1996 to 2014 Week 50
Search Title: Migraine Acute – L Richer – Update 2.0 | EMBASE – 19 Dec 2014 ‒ RF
Date Searched: 19 December 2014
Results:
| Migraine related terms: 1. exp Headache Disorders/ 2. vascular headache/ 3. headache/ 4. (migraine$ or headache$ or head‐ache$ or cephalgia or cephalalgia).ti,ab. 5. or/1‐4 (180,453) Pharmaceutical related terms: 6. exp Drug Therapy/ 7. (drug adj3 (therap$ or treatment?)).mp. 8. ((anti‐migrain$ or antimigrain$) adj3 (therap$ or treatment?)).mp. 9. (ad or ae or dt or to).fs. 10. exp Treatment Outcome/ 11. exp Analgesics/ 12. "nonsteroidal anti‐inflammatory agent?".mp. 13. "non‐steroidal anti‐inflammatory agent?".mp. 14. NSAID?.mp. 15. ibuprofen.mp. 16. fenoprofen.mp. 17. flurbiprofen.mp. 18. ketoprofen.mp. 19. ketorolac.mp. 20. diclofenac.mp. 21. etodolac.mp. 22. sulindac.mp. 23. diflunisal.mp. 24. naproxen.mp. 25. oxaprozin.mp. 26. tiaprofenic acid.mp. 27. mefenamic acid.mp. 28. indomethacin.mp. 29. tolmetin.mp. 30. celecoxib.mp. 31. meloxicam.mp. 32. piroxicam.mp. 33. tenoxicam.mp. 34. floctafenin$.mp. 35. nabumeton$.mp. 36. acetaminophen.mp. 37. paracetamol.mp. 38. ergot$ alkaloid?.mp. 39. ergotamin$.mp. 40. dihydroergotoxin$.mp. 41. dihydroergotamin$.mp. 42. DHE.mp. 43. ergoloid mesylates.mp. 44. methysergide.mp. 45. ziconotide.mp. 46. opioid$.mp. 47. opiate$.mp. 48. opium.mp. 49. meperidine.mp. 50. alfentan#l.mp. 51. fentan#l.mp. 52. rem#fentan#l.mp. 53. sufentan#l.mp. 54. levomethadyl.mp. 55. butorphanol.mp. 56. codein?.mp. 57. morphine.mp. 58. pentazocin$.mp. 59. (propoxyphen$ or dextro?propoxyphen$).mp. 60. nalbuphin$.mp. 61. hydromorphon$.mp. 62. oxycodon$.mp. 63. oxymorphon$.mp. 64. methadon$.mp. 65. butalbital.mp. 66. aspirin.mp. 67. acetylsalicylic acid.mp. 68. caffeine.mp. 69. "combination analgesic?".tw. 70. APAP.tw. 71. dichloralphenazone.mp. 72. isomethepten$.mp. 73. corticosteroid$.mp. 74. hydrocortisone.mp. 75. prednisolone.mp. 76. methylprednisolone.mp. 77. dexamethasone.mp. 78. tryptamin$.mp. 79. triptan?.mp. 80. sumatriptan.mp. 81. naratriptan.mp. 82. rizatriptan.mp. 83. zolmitriptan.mp. 84. almotriptan.mp. 85. eletriptan.mp. 86. frovatriptan.mp. 87. serotonin agonist?.mp. 88. ((5‐hydroxytryptamine or 5‐HT) adj2 agonist?).mp. 89. (antiemetic? or anti‐emetic?).mp. 90. (antinauseant? or anti‐nauseant?).mp. 91. chlorpromazine.mp. 92. prochlorperazine.mp. 93. perphenazine.mp. 94. trifluoperazine.mp. 95. (met#clopr#mide or metochlopramide).mp. 96. scopolamin$.mp. 97. dimenhydrinate.mp. 98. dronabinol.mp. 99. nabilon$.mp. 100. thiethylperazine.mp. 101. trimethobenzamide.mp. 102. ondansetron.mp. 103. granisetron.mp. 104. dolasetron.mp. 105. diphenhydramine.mp. 106. hydroxyzine.mp. 107. promethazine.mp. 108. Valproic Acid.mp. 109. valproate.mp. 110. divalproex sodium.mp. 111. Clonidine.mp. 112. fluid bolus.mp. 113. normal saline.mp. 114. magnesium.mp. 115. lidocaine.mp. 116. Botulinum Toxin Type A/ 117. botulinium toxin.mp. 118. botox.mp. 119. oxygen.mp. 120. placebo$.mp. 121. or/6‐120 [combination of all pharmaceutical treatments for migraine] (4,461,554) RCT filter: 122. random*.tw. 123. placebo*.mp. 124. double‐blind*.tw. 125. or/122‐124 [RCT filter from J Med Libr Assoc 2006] (938,236) Child related terms: 126. adolescent/ 127. child/ 128. newborn/ 129. exp Pediatrics/ 130. infant$.mp. 131. infancy.mp. 132. newborn$.mp. 133. baby.mp. 134. babies.mp. 135. neonat$.mp. 136. preterm$.mp. 137. prematur$.mp. 138. postmatur$.mp. 139. child$.mp. 140. kid.mp. 141. kids.mp. 142. toddler$.mp. 143. adolescen$.mp. 144. teen$.mp. 145. juvenile$.mp. 146. boy$.mp. 147. girl.mp. 148. girls.mp. 149. minor$.mp. 150. pubert$.mp. 151. pubescen$.mp. 152. pediatric$.mp. 153. paediatric$.mp. 154. peadiatric$.mp. 155. or/126‐154 [child filter as per original search] 156. and/5,121,125,155 [combination of migrain + drugs + RCT + child] (6,679) 157. 2014*.dp,em,yr. [date of publication, entry date, year of publication limits] (1,525,211) 158. ("201318" or "201319" or 20132* or 20133* or 20134* or 20135*).em. [entry date limit] (1,086,901) 159. (or/157,158) and 156 [application of date limits] (931) 160. (1996* or 1997* or 1998* or 1999* or 2000* or 2001* or 2002* or 2003* or 2004* or 2005* or 2006* or 2007* or 2008* or 2009* or 2010* or 2011* or 2012* or 2013* or 2014*).dp,em,yr 161. 159 not 160 (794) 162. remove duplicates from 161 (787) |
Search Summary (update after June 2008):
| Review | Number Retrieved | After Duplicate Removal | Update Search Date | Number Retrieved | After Duplicate Removal |
| MEDLINE | 639 | 616 | 29 April 2013 | 90 | 75 |
| MEDLINE In‐Process | 29 | 29 | 29 April 2013 | 16 | 14 |
| CCRT | 153 | 127 | 29 April 2013 | 10 | 2 |
| CDSR DARE | 664 | 650 | 29 April 2013 | 324 | 169 |
| IPA | 40 | 10 | 29 April 2013 | 7 | 4 |
| PsycINFO | 147 | 85 | 29 April 2013 | 5 | 3 |
| EMBASE | 2,884 | 2,511 | 30 April 2013 | 508 | 411 |
| CINAHL | 213 | 70 | 30 April 2013 | 31 | 21 |
| Total | 4769 | 4098 | 991 | 699 |
Database: MEDLINE via Ovid <1946 to Present>
Search Title: Migraine Acute ‒ L Richer ‒ Update 1.0 | MEDLINE ‒ 7 June 2012 ‒ AM
|
Migraine related terms: 1. exp Headache Disorders/ 2. vascular headaches/ 3. headache/ 4. (migraine$ or headache$ or head‐ache$ or cephalgia or cephalalgia).ti,ab. |
| 5. or/1‐4 (70,483) |
|
Pharmaceutical related terms: 6. exp Drug Therapy/ 7. (ad or ae or dt or to).fs. 8. exp Treatment Outcome/ 9. exp Analgesics/ 10. "nonsteroidal anti‐inflammatory agent?".mp. 11. "non‐steroidal anti‐inflammatory agent?".mp. 12. NSAID?.mp. 13. ibuprofen.mp. 14. fenoprofen.mp. 15. flurbiprofen.mp. 16. ketoprofen.mp. 17. ketorolac.mp. 18. diclofenac.mp. 19. etodolac.mp. 20. sulindac.mp. 21. naproxen.mp. 22. tolmetin.mp. 23. oxaprozin.mp. 24. tenoxicam.mp. 25. tiaprofenic acid.mp. 26. mefenamic acid.mp. 27. ((acetylsalicylic adj1.acid) or aspirin).mp. 28. piroxicam.mp. 29. celecoxib.mp. 30. meloxicam.mp. 31. indomethacin.mp. 32. floctafenin$.mp. 33. nabumeton$.mp. 34. acetaminophen.mp. 35. paracetamol.mp. 36. ergotamin$.mp. 37. dihydroergotamin$.mp. 38. DHE.mp. 39. opioid$.mp. 40. opium.mp. 41. methadon$.mp. 42. meperidine.mp. 43. butorphanol.mp. 44. hydromorphon$.mp. 45. morphin$.mp. 46. codein?.mp. 47. butalbital.mp. 48. pentazocine.mp. 49. propoxyphene.mp. 50. nalbuphine.mp. 51. oxycodon$.mp. 52. ocymorphon$.mp. 53. alfentanil.mp. 54. fentanyl.mp. 55. sufentanil.mp. 56. caffeine.mp. 57. "combination analgesic?".tw. 58. tryptamines.mp. 59. triptan?.mp. 60. sumatriptan.mp. 61. naratriptan.mp. 62. rizatriptan.mp. 63. zolmitriptan.mp. 64. almotriptan.mp. 65. eletriptan.mp. 66. frovatriptan.mp. 67. serotonin agonist?.mp. 68. ((5‐hydroxytriptamine or 5‐HT) adj2 agonist?).mp. 69. (antiemetic? or anti‐emetic?).mp. 70. (antinauseant? or anti‐nauseant?).mp. 71. thiethylperazin$.mp. 72. trimethobenzamid$.mp. 73. scopolamin$.mp. 74. chlorpromazine.mp. 75. prochlorperazine.mp. 76. promethazin$.mp. 77. perphenazin$.mp. 78. trifluoperazin$.mp. 79. met#clopr#mide.mp. 80. ondansetron.mp. 81. granisetron.mp. 82. dolasetron.mp. 83. diphenhydramine.mp. 84. hydroxyzine.mp. 85. dimenhydrinate.mp. 86. dronabinol.mp. 87. nabilone.mp. 88. fluid bolus.mp. 89. normal saline.mp. 90. magnesium.mp. 91. lidocaine.mp. 92. corticosteroid$.mp. 93. prednisolone.mp. 94. solumedrol.mp. 95. dexamethason$.mp. 96. hydrocortisol.mp. 97. exp methylprednisolone/ 98. Botulinum Toxin Type A/ 99. botulinium toxin.mp. 100. botox.mp. 101. oxygen.mp. 102. clonidine.mp. 103. diflunisal.mp. 104. (levomethadyl or levo‐methadyl).mp. 105. remifentanil.mp. 106. ziconotid$.mp. 107. placebo$.mp. 108. (antimigrain$ or anti‐migrain$).mp. 109. divalproex.mp. 110. methysergid$.mp. 111. (ergoloid adj1 mesylate$).mp. 112. (abortive adj3 (therap$ or treatment$)).mp. |
| 113. or/6‐112 (4,457,175) |
|
RCT filter: 114. and/5,113 (33,933) 115. randomized controlled trial.pt. 116. controlled clinical trial.pt. 117. randomized.ab. 118. placebo.ab. 119. clinical trials as topic.sh. 120. randomly.ab. 121. trial.ti. 122. or/115‐121 (762,426) 123. exp animals/ not humans.sh. (3,730,608) |
| 124. 122 not 123 [Updated Cochrane RCT filter max specificity and precision] (702,878) |
|
Child related terms: 125. exp Infant/ 126. exp Child/ 127. Adolescent/ 128. Minors/ 129. exp Puberty/ 130. exp Pediatrics/ 131. infant$.mp. 132. infancy.mp. 133. newborn$.mp. 134. baby.mp. 135. babies.mp. 136. neonat$.mp. 137. preterm$.mp. 138. prematur$.mp. 139. postmatur$.mp. 140. child$.mp. 141. kid.mp. 142. kids.mp. 143. toddler$.mp. 144. adolescen$.mp. 145. teen$.mp. 146. boy$.mp. 147. girl.mp. 148. minor$.mp. 149. pubert$.mp. 150. pubescen$.mp. 151. prepubescen$.mp. 152. pediatric$.mp. 153. paediatric$.mp. 154. peadiatric$.mp. |
| 155. or/125‐154 (3,241,093) |
| 156. and/114,124,155 [combination of migraine + drug terms + RCT filter + child filter] (2,844) |
|
Date limits for update: 157. limit 156 to ed="20080101‐20120630" (639) 158. (2008* or 2009* or 201*).dp,ep,yr. [date of publication (.dp), electronic date of entry (.ep) and year (.yr) search fields for update] (3,078,760) 159. and/156,158 [application of date restrictions to search results] (599) 160. or/157,159 [combination of entry date and other date restrictions] (639) |
|
Date limits for update 2013: 161. limit 156 to ed="20120630‐20130430" (90) 162. (2012 jun* or 2013*).dp,ep. [date of publication (.dp), electronic date of entry (.ep)] (95,823) 163. and/156,162 [application of date restrictions to search results] (12) 164. or/161,163 [combination of date restrictions] (90) |
Database: MEDLINE In‐Process via Ovid <June 12, 2012>
Search Title: Migraine Acute ‒ L Richer ‒ Update 3.0 | Keyword Search ‒ 8 June 2012 ‒ AM
|
Migraine related terms: 1. (migraine$ or headache$ or head‐ache$ or cephalgia or cephalalgia).mp. (3,432) |
|
Pharmaceutical related terms: 2. (drug adj3 (therap$ or treatment?)).mp. 3. ((anti‐migrain$ or antimigrain$) adj3 (therap$ or treatment?)).mp. 4. (treatment adj5.outcome).mp. 5. analgesi$.mp. 6. "nonsteroidal anti‐inflammatory agent?".mp. 7. "non‐steroidal anti‐inflammatory agent?".mp. 8. NSAID?.mp. 9. ibuprofen.mp. 10. fenoprofen.mp. 11. flurbiprofen.mp. 12. ketoprofen.mp. 13. ketorolac.mp. 14. diclofenac.mp. 15. etodolac.mp. 16. sulindac.mp. 17. diflunisal.mp. 18. naproxen.mp. 19. oxaprozin.mp. 20. tiaprofenic acid.mp. 21. mefenamic acid.mp. 22. indomethacin.mp. 23. tolmetin.mp. 24. celecoxib.mp. 25. meloxicam.mp. 26. piroxicam.mp. 27. tenoxicam.mp. 28. floctafenin$.mp. 29. nabumeton$.mp. 30. acetaminophen.mp. 31. paracetamol.mp. 32. ergot$ alkaloid?.mp. 33. ergotamin$.mp. 34. dihydroergotoxin$.mp. 35. dihydroergotamin$.mp. 36. DHE.mp. 37. ergoloid mesylates.mp. 38. methysergide.mp. 39. ziconotide.mp. 40. opioid$.mp. 41. opiate$.mp. 42. opium.mp. 43. meperidine.mp. 44. alfentan#l.mp. 45. fentan#l.mp. 46. rem#fentan#l.mp. 47. sufentan#l.mp. 48. levomethadyl.mp. 49. butorphanol.mp. 50. codein?.mp. 51. morphine.mp. 52. pentazocin$.mp. 53. (propoxyphen$ or dextro?propoxyphen$).mp. 54. nalbuphin$.mp. 55. hydromorphon$.mp. 56. oxycodon$.mp. 57. oxymorphon$.mp. 58. methadon$.mp. 59. butalbital.mp. 60. aspirin.mp. 61. acetylsalicylic acid.mp. 62. caffeine.mp. 63. "combination analgesic?".tw. 64. APAP.tw. 65. dichloralphenazone.mp. 66. isomethepten$.mp. 67. corticosteroid$.mp. 68. hydrocortisone.mp. 69. prednisolone.mp. 70. methylprednisolone.mp. 71. dexamethasone.mp. 72. tryptamin$.mp. 73. triptan?.mp. 74. sumatriptan.mp. 75. naratriptan.mp. 76. rizatriptan.mp. 77. zolmitriptan.mp. 78. almotriptan.mp. 79. eletriptan.mp. 80. frovatriptan.mp. 81. serotonin agonist?.mp. 82. ((5‐hydroxytryptamine or 5‐HT) adj2 agonist?).mp. 83. (antiemetic? or anti‐emetic?).mp. 84. (antinauseant? or anti‐nauseant?).mp. 85. chlorpromazine.mp. 86. prochlorperazine.mp. 87. perphenazine.mp. 88. trifluoperazine.mp. 89. (met#clopr#mide or metochlopramide).mp. 90. scopolamin$.mp. 91. dimenhydrinate.mp. 92. dronabinol.mp. 93. nabilon$.mp. 94. thiethylperazine.mp. 95. trimethobenzamide.mp. 96. ondansetron.mp. 97. granisetron.mp. 98. dolasetron.mp. 99. diphenhydramine.mp. 100. hydroxyzine.mp. 101. promethazine.mp. 102. Valproic Acid.mp. 103. valproate.mp. 104. divalproex sodium.mp. 105. Clonidine.mp. 106. fluid bolus.mp. 107. normal saline.mp. 108. magnesium.mp. 109. lidocaine.mp. 110. botulinium toxin.mp. 111. botox.mp. 112. oxygen.mp. 113. placebo$.mp. |
| 114. or/2‐113 [migraine drugs] (50,953) |
|
RCT filter: 115. randomized controlled trial.pt. 116. controlled clinical trial.pt. 117. randomized.ab. 118. placebo.ab. 119. randomly.ab. 120. trial.ti. 121. or/115‐120 (27,679) 122. exp animals/ not humans.sh. (1) |
| 123. 121.not 122 [Cochrane RCT filter, slightly modified for database] (27,679) |
| 124. and/1,114,123 [combination of migraine + drugs + RCT terms] (233) |
|
Child related terms: 125. infant$.mp. 126. infancy.mp. 127. newborn$.mp. 128. baby.mp. 129. babies.mp. 130. neonat$.mp. 131. preterm$.mp. 132. prematur$.mp. 133. postmatur$.mp. 134. child$.mp. 135. kid.mp. 136. kids.mp. 137. toddler$.mp. 138. adolescen$.mp. 139. teen$.mp. 140. boy$.mp. 141. girl.mp. 142. girls.mp. 143. minor$.mp. 144. pubert$.mp. 145. pubescen$.mp. 146. pediatric$.mp. 147. paediatric$.mp. 148. peadiatric$.mp. |
| 149. or/125‐148 [child filter] (66,612) |
| 150. and/124,149 [migraine + drug + RCT + child] (29) |
|
Date limits for update: 151. (2008* or 2009* or 201*).ed,ep,up,yr. [entry date, e‐pub date, update code, year of pub] (1,343,461) 152. 150 and 151 (29) |
|
Date limits for update 2013: 153. ("2012" or "2013").ed,ep,up,yr. [entry date, e‐pub date, update code, year of pub] (471,980) 154. and/150,153 (16) |
Database Searched: Evidence Based Medicine Reviews via Ovid: Cochrane Central Register of Controlled Trials < May 2012 >
Search Title: Migraine Acute ‒ L Richer ‒ Update 1.1 | Cochrane ‒ no SD filters ‒ 11 June 2012 ‒ AM
|
Migraine related terms: 1. exp Headache Disorders/ 2. vascular headaches/ 3. headache/ 4. (migraine$ or headache$ or head‐ache$ or cephalgia or cephalalgia).ti,ab. |
| 5. or/1‐4 (8,180) |
|
Pharmaceutical related terms: 6. exp Drug Therapy/ 7. (ad or ae or dt or to).fs. 8. exp Treatment Outcome/ 9. exp Analgesics/ 10. "nonsteroidal anti‐inflammatory agent?".mp. 11. "non‐steroidal anti‐inflammatory agent?".mp. 12. NSAID?.mp. 13. ibuprofen.mp. 14. fenoprofen.mp. 15. flurbiprofen.mp. 16. ketoprofen.mp. 17. ketorolac.mp. 18. diclofenac.mp. 19. etodolac.mp. 20. sulindac.mp. 21. naproxen.mp. 22. tolmetin.mp. 23. oxaprozin.mp. 24. tenoxicam.mp. 25. tiaprofenic acid.mp. 26. mefenamic acid.mp. 27. ((acetylsalicylic adj1 acid) or aspirin).mp. 28. piroxicam.mp. 29. celecoxib.mp. 30. meloxicam.mp. 31. indomethacin.mp. 32. floctafenin$.mp. 33. nabumeton$.mp. 34. acetaminophen.mp. 35. paracetamol.mp. 36. ergotamin$.mp. 37. dihydroergotamin$.mp. 38. DHE.mp. 39. opioid$.mp. 40. opium.mp. 41. methadon$.mp. 42. meperidine.mp. 43. butorphanol.mp. 44. hydromorphon$.mp. 45. morphin$.mp. 46. codein?.mp. 47. butalbital.mp. 48. pentazocine.mp. 49. propoxyphene.mp. 50. nalbuphine.mp. 51. oxycodon$.mp. 52. ocymorphon$.mp. 53. alfentanil.mp. 54. fentanyl.mp. 55. sufentanil.mp. 56. caffeine.mp. 57. "combination analgesic?".tw. 58. tryptamines.mp. 59. triptan?.mp. 60. sumatriptan.mp. 61. naratriptan.mp. 62. rizatriptan.mp. 63. zolmitriptan.mp. 64. almotriptan.mp. 65. eletriptan.mp. 66. frovatriptan.mp. 67. serotonin agonist?.mp. 68. ((5‐hydroxytriptamine or 5‐HT) adj2 agonist?).mp. 69. (antiemetic? or anti‐emetic?).mp. 70. (antinauseant? or anti‐nauseant?).mp. 71. thiethylperazin$.mp. 72. trimethobenzamid$.mp. 73. scopolamin$.mp. 74. chlorpromazine.mp. 75. prochlorperazine.mp. 76. promethazin$.mp. 77. perphenazin$.mp. 78. trifluoperazin$.mp. 79. met#clopr#mide.mp. 80. ondansetron.mp. 81. granisetron.mp. 82. dolasetron.mp. 83. diphenhydramine.mp. 84. hydroxyzine.mp. 85. dimenhydrinate.mp. 86. dronabinol.mp. 87. nabilone.mp. 88. fluid bolus.mp. 89. normal saline.mp. 90. magnesium.mp. 91. lidocaine.mp. 92. corticosteroid$.mp. 93. prednisolone.mp. 94. solumedrol.mp. 95. dexamethason$.mp. 96. hydrocortisol.mp. 97. exp methylprednisolone/ 98. Botulinum Toxin Type A/ 99. botulinium toxin.mp. 100. botox.mp. 101. oxygen.mp. 102. clonidine.mp. 103. diflunisal.mp. 104. (levomethadyl or levo‐methadyl).mp. 105. remifentanil.mp. 106. ziconotid$.mp. 107. placebo$.mp. 108. (antimigrain$ or anti‐migrain$).mp. 109. divalproex.mp. 110. methysergid$.mp. 111. (ergoloid adj1 mesylate$).mp. 112. (abortive adj3 (therap$ or treatment$)).mp. |
| 113. or/6‐112 (335,914) |
| 114. and/5,113 (6,616) |
|
Child related terms: 115. exp Infant/ 116. exp Child/ 117. Adolescent/ 118. Minors/ 119. exp Puberty/ 120. exp Pediatrics/ 121. infant$.mp. 122. infancy.mp. 123. newborn$.mp. 124. baby.mp. 125. babies.mp. 126. neonat$.mp. 127. preterm$.mp. 128. prematur$.mp. 129. postmatur$.mp. 130. child$.mp. 131. kid.mp. 132. kids.mp. 133. toddler$.mp. 134. adolescen$.mp. 135. teen$.mp. 136. boy$.mp. 137. girl.mp. 138. minor$.mp. 139. pubert$.mp. 140. pubescen$.mp. 141. prepubescen$.mp. 142. pediatric$.mp. 143. paediatric$.mp. 144. peadiatric$.mp. |
| 145. or/115‐144 (136,786) |
| 146. and/114,145 [migraine + drugs + child] (2,107) |
|
Removal of MEDLINE records: 147. limit 146 to medline records (1,954) 148. 146 not 147 (153) |
|
Date limits for update: 149. (2008* or 2009* or 201*).up. (671,470) 150. and/148‐149 [application of update code limit] (153) |
| 151. limit 148 to latest update (2) 152. (2012 jun* or 2013*).up. [update code limit] (27,314) 153. new.uf. [update flag for new articles] (30,970) 154. (152 or 153) and 148 [application of update code and update flag to results] (10) 155. or/151,154 [combination of update results] (10) |
Database Searched: Evidence Based Medicine Reviews via Ovid
Cochrane Database of Systematic Reviews <20(2005 to May 2012>, Database of Abstracts of Reviews of Effects <2nd Quarter 2012>
Search Title: Migraine Acute ‒ L Richer ‒ Update 3.2 | CCRT ‒ 11 June 2012 ‒ AM
|
Migraine related terms: 1. (migraine$ or headache$ or head‐ache$ or cephalgia or cephalalgia).mp. (1,490) |
|
Pharmaceutical related terms: 2. (drug adj3 (therap$ or treatment?)).mp. 3. ((anti‐migrain$ or antimigrain$) adj3 (therap$ or treatment?)).mp. 4. (treatment adj5 outcome).mp. 5. analgesi$.mp. 6. "nonsteroidal anti‐inflammatory agent?".mp. 7. "non‐steroidal anti‐inflammatory agent?".mp. 8. NSAID?.mp. 9. ibuprofen.mp. 10. fenoprofen.mp. 11. flurbiprofen.mp. 12. ketoprofen.mp. 13. ketorolac.mp. 14. diclofenac.mp. 15. etodolac.mp. 16. sulindac.mp. 17. diflunisal.mp. 18. naproxen.mp. 19. oxaprozin.mp. 20. tiaprofenic acid.mp. 21. mefenamic acid.mp. 22. indomethacin.mp. 23. tolmetin.mp. 24. celecoxib.mp. 25. meloxicam.mp. 26. piroxicam.mp. 27. tenoxicam.mp. 28. floctafenin$.mp. 29. nabumeton$.mp. 30. acetaminophen.mp. 31. paracetamol.mp. 32. ergot$ alkaloid?.mp. 33. ergotamin$.mp. 34. dihydroergotoxin$.mp. 35. dihydroergotamin$.mp. 36. DHE.mp. 37. ergoloid mesylates.mp. 38. methysergide.mp. 39. ziconotide.mp. 40. opioid$.mp. 41. opiate$.mp. 42. opium.mp. 43. meperidine.mp. 44. alfentan#l.mp. 45. fentan#l.mp. 46. rem#fentan#l.mp. 47. sufentan#l.mp. 48. levomethadyl.mp. 49. butorphanol.mp. 50. codein?.mp. 51. morphine.mp. 52. pentazocin$.mp. 53. (propoxyphen$ or dextro?propoxyphen$).mp. 54. nalbuphin$.mp. 55. hydromorphon$.mp. 56. oxycodon$.mp. 57. oxymorphon$.mp. 58. methadon$.mp. 59. butalbital.mp. 60. aspirin.mp. 61. acetylsalicylic acid.mp. 62. caffeine.mp. 63. "combination analgesic?".tw. 64. APAP.tw. 65. dichloralphenazone.mp. 66. isomethepten$.mp. 67. corticosteroid$.mp. 68. hydrocortisone.mp. 69. prednisolone.mp. 70. methylprednisolone.mp. 71. dexamethasone.mp. 72. tryptamin$.mp. 73. triptan?.mp. 74. sumatriptan.mp. 75. naratriptan.mp. 76. rizatriptan.mp. 77. zolmitriptan.mp. 78. almotriptan.mp. 79. eletriptan.mp. 80. frovatriptan.mp. 81. serotonin agonist?.mp. 82. ((5‐hydroxytryptamine or 5‐HT) adj2 agonist?).mp. 83. (antiemetic? or anti‐emetic?).mp. 84. (antinauseant? or anti‐nauseant?).mp. 85. chlorpromazine.mp. 86. prochlorperazine.mp. 87. perphenazine.mp. 88. trifluoperazine.mp. 89. (met#clopr#mide or metochlopramide).mp. 90. scopolamin$.mp. 91. dimenhydrinate.mp. 92. dronabinol.mp. 93. nabilon$.mp. 94. thiethylperazine.mp. 95. trimethobenzamide.mp. 96. ondansetron.mp. 97. granisetron.mp. 98. dolasetron.mp. 99. diphenhydramine.mp. 100. hydroxyzine.mp. 101. promethazine.mp. 102. Valproic Acid.mp. 103. valproate.mp. 104. divalproex sodium.mp. 105. Clonidine.mp. 106. fluid bolus.mp. 107. normal saline.mp. 108. magnesium.mp. 109. lidocaine.mp. 110. botulinium toxin.mp. 111. botox.mp. 112. oxygen.mp. 113. placebo$.mp. |
| 114. or/2‐113 [migraine drugs] (16,264) |
| 115. and/1,114 [combination of migraine + drugs] (1,414) |
|
Child related terms: 116. infant$.mp. 117. infancy.mp. 118. newborn$.mp. 119. baby.mp. 120. babies.mp. 121. neonat$.mp. 122. preterm$.mp. 123. prematur$.mp. 124. postmatur$.mp. 125. child$.mp. 126. kid.mp. 127. kids.mp. 128. toddler$.mp. 129. adolescen$.mp. 130. teen$.mp. 131. boy$.mp. 132. girl.mp. 133. girls.mp. 134. minor$.mp. 135. pubert$.mp. 136. pubescen$.mp. 137. pediatric$.mp. 138. paediatric$.mp. 139. peadiatric$.mp. |
| 140. or/116‐139 [child filter] (9,102) |
| 141. and/115,140 [migraine + drug + RCT + child] (898) |
| 142. remove duplicates from 141 (898) |
|
Removal of articles which are protocols only: 143. limit 142 to protocols [Limit not valid in DARE; records were retained] (234) 144. 142 not 143 (664) |
|
Date limits for update: 145. limit 144to last 5years (493) 146. (2008* or 2009* or 201*).up. (7,498) 147. and/144,146 (664) 148. 145 or 147 (664) |
|
Date limits for update 2013: 149. (2012 jun* or 2013*).up. [update code limit] (859) 150. new.uf. [update flag for new articles] (4,557) 151. (149 or 150) and 144 [application of update codes to original results] (324) |
Database: International Pharmaceutical Abstracts via Ovid < 1970 to May 2012 >
Search Title: Migraine Acute ‒ L Richer ‒ Update 3.1 | IPA ‒ Keyword w/o pt terms ‒ 11 June 2012 ‒ AM
|
Migraine related terms: 1. (migraine$ or headache$ or head‐ache$ or cephalgia or cephalalgia).mp. (3,917) |
|
Pharmaceutical related terms: 2. (drug adj3 (therap$ or treatment?)).mp. 3. ((anti‐migrain$ or antimigrain$) adj3 (therap$ or treatment?)).mp. 4. (treatment adj5 outcome).mp. 5. analgesi$.mp. 6. "nonsteroidal anti‐inflammatory agent?".mp. 7. "non‐steroidal anti‐inflammatory agent?".mp. 8. NSAID?.mp. 9. ibuprofen.mp. 10. fenoprofen.mp. 11. flurbiprofen.mp. 12. ketoprofen.mp. 13. ketorolac.mp. 14. diclofenac.mp. 15. etodolac.mp. 16. sulindac.mp. 17. diflunisal.mp. 18. naproxen.mp. 19. oxaprozin.mp. 20. tiaprofenic acid.mp. 21. mefenamic acid.mp. 22. indomethacin.mp. 23. tolmetin.mp. 24. celecoxib.mp. 25. meloxicam.mp. 26. piroxicam.mp. 27. tenoxicam.mp. 28. floctafenin$.mp. 29. nabumeton$.mp. 30. acetaminophen.mp. 31. paracetamol.mp. 32. ergot$ alkaloid?.mp. 33. ergotamin$.mp. 34. dihydroergotoxin$.mp. 35. dihydroergotamin$.mp. 36. DHE.mp. 37. ergoloid mesylates.mp. 38. methysergide.mp. 39. ziconotide.mp. 40. opioid$.mp. 41. opiate$.mp. 42. opium.mp. 43. meperidine.mp. 44. alfentan#l.mp. 45. fentan#l.mp. 46. rem#fentan#l.mp. 47. sufentan#l.mp. 48. levomethadyl.mp. 49. butorphanol.mp. 50. codein?.mp. 51. morphine.mp. 52. pentazocin$.mp. 53. (propoxyphen$ or dextro?propoxyphen$).mp. 54. nalbuphin$.mp. 55. hydromorphon$.mp. 56. oxycodon$.mp. 57. oxymorphon$.mp. 58. methadon$.mp. 59. butalbital.mp. 60. aspirin.mp. 61. acetylsalicylic acid.mp. 62. caffeine.mp. 63."combination analgesic?".tw. 64. APAP.tw. 65. dichloralphenazone.mp. 66. isomethepten$.mp. 67. corticosteroid$.mp. 68. hydrocortisone.mp. 69. prednisolone.mp. 70. methylprednisolone.mp. 71. dexamethasone.mp. 72. tryptamin$.mp. 73. triptan?.mp. 74. sumatriptan.mp. 75. naratriptan.mp. 76. rizatriptan.mp. 77. zolmitriptan.mp. 78. almotriptan.mp. 79. eletriptan.mp. 80. frovatriptan.mp. 81. serotonin agonist?.mp. 82. ((5‐hydroxytryptamine or 5‐HT) adj2 agonist?).mp. 83. (antiemetic? or anti‐emetic?).mp. 84. (antinauseant? or anti‐nauseant?).mp. 85. chlorpromazine.mp. 86. prochlorperazine.mp. 87. perphenazine.mp. 88. trifluoperazine.mp. 89. (met#clopr#mide or metochlopramide).mp. 90. scopolamin$.mp. 91. dimenhydrinate.mp. 92. dronabinol.mp. 93. nabilon$.mp. 94. thiethylperazine.mp. 95. trimethobenzamide.mp. 96. ondansetron.mp. 97. granisetron.mp. 98. dolasetron.mp. 99. diphenhydramine.mp. 100. hydroxyzine.mp. 101. promethazine.mp. 102. Valproic Acid.mp. 103. valproate.mp. 104. divalproex sodium.mp. 105. Clonidine.mp. 106. fluid bolus.mp. 107. normal saline.mp. 108. magnesium.mp. 109. lidocaine.mp. 110. botulinium toxin.mp. 111. botox.mp. 112. oxygen.mp. 113. placebo$.mp. |
| 114. or/2‐113 [migraine drugs] (97,647) |
|
RCT filter: 115. randomized.ab. 116. placebo.ab. 117. randomly.ab. 118. trial*.tw. |
| 119. or/115‐118 (59,621) |
| 120. and/1,114,119 [combination of migraine + drugs + RCT terms] (1,011) |
|
Child related terms: 121. infant$.mp. 122. infancy.mp. 123. newborn$.mp. 124. baby.mp. 125. babies.mp. 126. neonat$.mp. 127. preterm$.mp. 128. prematur$.mp. 129. postmatur$.mp. 130. child$.mp. 131. kid.mp. 132. kids.mp. 133. toddler$.mp. 134. adolescen$.mp. 135. teen$.mp. 136. boy$.mp. 137. girl.mp. 138. girls.mp. 139. minor$.mp. 140. pubert$.mp. 141. pubescen$.mp. 142. pediatric$.mp. 143. paediatric$.mp. 144. peadiatric$.mp. |
| 145. or/121‐144 [child filter] (36,856) |
| 146. and/120,145 [migraine + drug + RCT + child] (109) |
|
Date limits for update: 147. (2008* or 2009* or 201*).ed,ep,up,yr. [entry date, e‐pub date, update code, year of pub] (87,846) 148. 146 and 147 (40) |
| 149. (2012* or 2013*).em. [entry month date limit] (27,810) 150. and/146,149 [application of entry month date limit] (7) |
Database: PsycINFO via Ovid < 1806 to June Week 1 2012 >
Search Title: Migraine Acute ‒ L Richer ‒ Update 4.0 | PsycINFO ‒ 12 June 2012 ‒ AM
|
Migraine related terms: 1. headache/ 2. migraine/ 3. muscle contraction headache/ 4. (migraine$ or headache$ or head‐ache$ or cephalgia or cephalalgia).ti,ab. |
| 5. or/1‐4 (15,618) |
|
Pharmaceutical related terms: 6. Drug Therapy/ 7. exp drugs/ 8. exp "side effects (drug)"/ 9. (drug adj3 (therap$ or treatment?)).mp. 10. ((anti‐migrain$ or antimigrain$) adj3 (therap$ or treatment?)).mp. 11. treatment outcomes/ 12. (treatment adj5 outcome).mp. 13. analgesi$.mp. 14. "nonsteroidal anti‐inflammatory agent?".mp. 15. "non‐steroidal anti‐inflammatory agent?".mp. 16. NSAID?.mp. 17. ibuprofen.mp. 18. fenoprofen.mp. 19. flurbiprofen.mp. 20. ketoprofen.mp. 21. ketorolac.mp. 22. diclofenac.mp. 23. etodolac.mp. 24. sulindac.mp. 25. diflunisal.mp. 26. naproxen.mp. 27. oxaprozin.mp. 28. tiaprofenic acid.mp. 29. mefenamic acid.mp. 30. indomethacin.mp. 31. tolmetin.mp. 32. celecoxib.mp. 33. meloxicam.mp. 34. piroxicam.mp. 35. tenoxicam.mp. 36. floctafenin$.mp. 37. nabumeton$.mp. 38. acetaminophen.mp. 39. paracetamol.mp. 40. ergot$ alkaloid?.mp. 41. ergotamin$.mp. 42. dihydroergotoxin$.mp. 43. dihydroergotamin$.mp. 44. DHE.mp. 45. ergoloid mesylates.mp. 46. methysergide.mp. 47. ziconotide.mp. 48. opioid$.mp. 49. opiate$.mp. 50. opium.mp. 51. meperidine.mp. 52. alfentan#l.mp. 53. fentan#l.mp. 54. rem#fentan#l.mp. 55. sufentan#l.mp. 56. levomethadyl.mp. 57. butorphanol.mp. 58. codein?.mp. 59. morphine.mp. 60. pentazocin$.mp. 61. (propoxyphen$ or dextro?propoxyphen$).mp. 62. nalbuphin$.mp. 63. hydromorphon$.mp. 64. oxycodon$.mp. 65. oxymorphon$.mp. 66. methadon$.mp. 67. butalbital.mp. 68. aspirin.mp. 69. acetylsalicylic acid.mp. 70. caffeine.mp. 71. "combination analgesic?".tw. 72. APAP.tw. 73. dichloralphenazone.mp. 74. isomethepten$.mp. 75. corticosteroid$.mp. 76. hydrocortisone.mp. 77. prednisolone.mp. 78. methylprednisolone.mp. 79. dexamethasone.mp. 80. tryptamin$.mp. 81. triptan?.mp. 82. sumatriptan.mp. 83. naratriptan.mp. 84. rizatriptan.mp. 85. zolmitriptan.mp. 86. almotriptan.mp. 87. eletriptan.mp. 88. frovatriptan.mp. 89. serotonin agonist?.mp. 90. ((5‐hydroxytryptamine or 5‐HT) adj2 agonist?).mp. 91. (antiemetic? or anti‐emetic?).mp. 92. (antinauseant? or anti‐nauseant?).mp. 93. chlorpromazine.mp. 94. prochlorperazine.mp. 95. perphenazine.mp. 96. trifluoperazine.mp. 97. (met#clopr#mide or metochlopramide).mp. 98. scopolamin$.mp. 99. dimenhydrinate.mp. 100. dronabinol.mp. 101. nabilon$.mp. 102. thiethylperazine.mp. 103. trimethobenzamide.mp. 104. ondansetron.mp. 105. granisetron.mp. 106. dolasetron.mp. 107. diphenhydramine.mp. 108. hydroxyzine.mp. 109. promethazine.mp. 110. Valproic Acid.mp. 111. valproate.mp. 112. divalproex sodium.mp. 113. Clonidine.mp. 114. fluid bolus.mp. 115. normal saline.mp. 116. magnesium.mp. 117. lidocaine.mp. 118. botulinium toxin.mp. 119. botox.mp. 120. oxygen.mp. 121. placebo$.mp. 122. or/6‐121 [drug terms] (310,358) |
|
RCT filter: 123. double‐blind.tw. 124. random* assigned.tw. 125. control*.tw. |
| 126. or/123‐125 [HIRU max sensivity/specificity for RCTs] (439,506) |
| 127. and/5,122,126 [sensivity/specificity filter] (1,981) |
|
Child related terms: 128. infant$.mp. 129. infancy.mp. 130. newborn$.mp. 131. baby.mp. 132. babies.mp. 133. neonat$.mp. 134. preterm$.mp. 135. prematur$.mp. 136. postmatur$.mp. 137. child$.mp. 138. kid.mp. 139. kids.mp. 140. toddler$.mp. 141. adolescen$.mp. 142. teen$.mp. 143. boy$.mp. 144. girl.mp. 145. girls.mp. 146. minor$.mp. 147. pubert$.mp. 148. pubescen$.mp. 149. pediatric$.mp. 150. paediatric$.mp. 151. peadiatric$.mp. |
| 152. or/128‐151 (717,968) |
| 153. and/127,152 [sensitivity/specificity filter] (255) |
|
Date limits for update: 154. limit 153 to yr="2008‐Current" (107) 155. (2008* or 2009* or 201*).yr,dp,up. [year of publication, date of publication, update code search] (803,770) |
| 156. and/153,155 [application of date limits] (147) 157. or/154,156 [ORing both date limits] (147) |
| 158. (2012 jun* or 2013*).dp,up. [date of publication, update code search] (62,816) 159. and/153,158 [application of date limits] (5) |
Database: EMBASE via Ovid <1980 to present>
Search Title: Migraine Acute ‒ L Richer ‒ Update 2.0 | EMBASE ‒ 8 June 2012 ‒ AM
|
Migraine related terms: 1. exp Headache Disorders/ 2. vascular headache/ 3. headache/ 4. (migraine$ or headache$ or head‐ache$ or cephalgia or cephalalgia).ti,ab. |
| 5. or/1‐4 (188,282) |
|
Pharmaceutical related terms: 6. exp Drug Therapy/ 7. (drug adj3 (therap$ or treatment?)).mp. 8. ((anti‐migrain$ or antimigrain$) adj3 (therap$ or treatment?)).mp. 9. (ad or ae or dt or to).fs. 10. exp Treatment Outcome/ 11. exp Analgesics/ 12. "nonsteroidal anti‐inflammatory agent?".mp. 13. "non‐steroidal anti‐inflammatory agent?".mp. 14. NSAID?.mp. 15. ibuprofen.mp. 16. fenoprofen.mp. 17. flurbiprofen.mp. 18. ketoprofen.mp. 19. ketorolac.mp. 20. diclofenac.mp. 21. etodolac.mp. 22. sulindac.mp. 23. diflunisal.mp. 24. naproxen.mp. 25. oxaprozin.mp. 26. tiaprofenic acid.mp. 27. mefenamic acid.mp. 28. indomethacin.mp. 29. tolmetin.mp. 30. celecoxib.mp. 31. meloxicam.mp. 32. piroxicam.mp. 33. tenoxicam.mp. 34. floctafenin$.mp. 35. nabumeton$.mp. 36. acetaminophen.mp. 37. paracetamol.mp. 38. ergot$ alkaloid?.mp. 39. ergotamin$.mp. 40. dihydroergotoxin$.mp. 41. dihydroergotamin$.mp. 42. DHE.mp. 43. ergoloid mesylates.mp. 44. methysergide.mp. 45. ziconotide.mp. 46. opioid$.mp. 47. opiate$.mp. 48. opium.mp. 49. meperidine.mp. 50. alfentan#l.mp. 51. fentan#l.mp. 52. rem#fentan#l.mp. 53. sufentan#l.mp. 54. levomethadyl.mp. 55. butorphanol.mp. 56. codein?.mp. 57. morphine.mp. 58. pentazocin$.mp. 59. (propoxyphen$ or dextro?propoxyphen$).mp. 60. nalbuphin$.mp. 61. hydromorphon$.mp. 62. oxycodon$.mp. 63. oxymorphon$.mp. 64. methadon$.mp. 65. butalbital.mp. 66. aspirin.mp. 67. acetylsalicylic acid.mp. 68. caffeine.mp. 69. "combination analgesic?".tw. 70. APAP.tw. 71. dichloralphenazone.mp. 72. isomethepten$.mp. 73. corticosteroid$.mp. 74. hydrocortisone.mp. 75. prednisolone.mp. 76. methylprednisolone.mp. 77. dexamethasone.mp. 78. tryptamin$.mp. 79. triptan?.mp. 80. sumatriptan.mp. 81. naratriptan.mp. 82. rizatriptan.mp. 83. zolmitriptan.mp. 84. almotriptan.mp. 85. eletriptan.mp. 86. frovatriptan.mp. 87. serotonin agonist?.mp. 88. ((5‐hydroxytryptamine or 5‐HT) adj2 agonist?).mp. 89. (antiemetic? or anti‐emetic?).mp. 90. (antinauseant? or anti‐nauseant?).mp. 91. chlorpromazine.mp. 92. prochlorperazine.mp. 93. perphenazine.mp. 94. trifluoperazine.mp. 95. (met#clopr#mide or metochlopramide).mp. 96. scopolamin$.mp. 97. dimenhydrinate.mp. 98. dronabinol.mp. 99. nabilon$.mp. 100. thiethylperazine.mp. 101. trimethobenzamide.mp. 102. ondansetron.mp. 103. granisetron.mp. 104. dolasetron.mp. 105. diphenhydramine.mp. 106. hydroxyzine.mp. 107. promethazine.mp. 108. Valproic Acid.mp. 109. valproate.mp. 110. divalproex sodium.mp. 111. Clonidine.mp. 112. fluid bolus.mp. 113. normal saline.mp. 114. magnesium.mp. 115. lidocaine.mp. 116. Botulinum Toxin Type A/ 117. botulinium toxin.mp. 118. botox.mp. 119. oxygen.mp. 120. placebo$.mp. |
| 121. or/6‐120 [combination of all possible pharmaceutical treatments for migraine] (5,743,355) |
|
RCT filter: 122. random*.tw. 123. placebo*.mp. 124. double‐blind*.tw. |
| 125. or/122‐124 [RCT filter from J Med Libr Assoc 2006] (907,731) |
|
Child related terms: 126. adolescent/ 127. child/ 128. newborn/ 129. exp Pediatrics/ 130. infant$.mp. 131. infancy.mp. 132. newborn$.mp. 133. baby.mp. 134. babies.mp. 135. neonat$.mp. 136. preterm$.mp. 137. prematur$.mp. 138. postmatur$.mp. 139. child$.mp. 140. kid.mp. 141. kids.mp. 142. toddler$.mp. 143. adolescen$.mp. 144. teen$.mp. 145. juvenile$.mp. 146. boy$.mp. 147. girl.mp. 148. girls.mp. 149. minor$.mp. 150. pubert$.mp. 151. pubescen$.mp. 152. pediatric$.mp. 153. paediatric$.mp. 154. peadiatric$.mp. |
| 155. or/126‐154 [child filter as per original search] |
| 156. and/5,121,125,155 [combination of migrain + drugs + RCT + child] (6,357) |
| 157. (2008* or 2009* or 201*).dp,em,yr. [date of publication, entry date, year of publication limits] (4,936,810) 158. and/156‐157 [application of date limits] (2,884) |
|
Update search limits for 2013: 159. (2012 jun* or 2013*).dp. [date of publication limit] (24,334) 160. (20122* or 20123* or 20124* or 20125* or 2013*).em. [entry date limit] (1,247,362) 161. (or/159‐160) and 156 [application of date limits] (508) |
Database: CINAHL Plus with Full Text via Ebsco <1937 to present>
Search Title: Pediatric Migraine ‒ CINAHL ‒ Update ‒ 12 June 2012 ‒ AM
| Limiters ‐ Published Date from: 20120601‐20130531 (31) |
| S17=S16 Limiters ‐ Published Date from: 20080101‐20120631 (263) |
| S16=S1 and S2 and S14 and S15 (712) |
|
Child related terms: S15=((MH "Infant+") or (MH "Child+") or (MH "Adolescence") or (MH "Puberty+") or (MH "Pediatrics+") or infant* or infanc* or newborn* or baby or neonat* or preterm* or prematur* or postmatur* or child* or kid or kids or toddler* or adolescen* or teen* or boy* or girl or girls* or minor* or pubert* or pubescen* or pediatric* or paediatric* or peadiatric*) (601,431) |
| S14=S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 (807,111) |
|
RCT filter: S13=TX allocat* random* S12=MH "Quantitative Studies" S11=(MH "Placebos") S10=TX placebo* S9=TX random* allocat* S8=(MH "Random Assignment") S7=TX randomi* control* trial* S6=TX ((singl* n1 blind*) or (singl* n1 mask*)) or TX ((doubl* n1 blind*) or (doubl* n1 mask*)) or TX ((tripl* n1 blind*) or (tripl* n1 mask*)) or TX ((trebl* n1 blind*) or (trebl* n1 mask*)) S5=TX clinic* n1 trial* S4=PT Clinical trial S3=(MH "Clinical Trials+") |
|
Pharmaceutical related terms: S2=( (MH "Drug Therapy+") or (MH "Drug Therapy, Combination+") or (MH "Drug Combinations+") or (MH "Drug Therapy+") or MH "Drug Therapy, Combination+" or MH "Drug Combinations+" or drug w3 therap* or drug w3 treatment* or anti‐migrain* w3 therap* or antimigrain* w3 treatment* or (MH "Treatment Outcomes+") or (MH "Analgesics+") or "nonsteroidal anti‐inflammatory agent?" or "non‐steroidal anti‐inflammatory agent?" or ibuprofen or fenoprofen or flurbiprofen or Ketorolac or Diclofenac or Etodolac or Sulindac or Diflunisal or Naproxen or Oxaprozin or "tiaprofenic acid" or "mefenamic acid" or Indomethacin or Tolmetin or Celecoxib ) or ( Meloxicam or Piroxicam or Tenoxicam or Floctafenin* or nabumeton* or acetaminophen or ergot* w1 alkaloid* or ergotamin* or dihydroergotoxin* or dihydroergotamin* or DHE or ergoloid w1 mesylates or methysergide or ziconotide or opioid* or opiate* ) or ( opium or alfentan?l or fentan?l or rem?fentan?l or sufentan?l or levomethadyl or butorphanol or codein* or morphine or pentazocin* or propoxyphen* or dextro‐propoxyphen* or dextropropoxyphen* or nalbuphin* or hydromorphon* or oxycodon* ) (425780) |
|
Migraine related terms: S1=( MH "Headache+" or MH "Vascular Headache+" ) or TI ( migraine* or headache* or head‐ache* or cephalgia or cephalalgia ) or AB ( migraine* or headache* or head‐ache* or cephalgia or cephalalgia ) (18,833) |
Data and analyses
Comparison 1. Ibuprofen vs placebo in children.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Pain‐free | 2 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [1.15, 3.04] |
| 1.2 Adverse events (any) | 1 | 80 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.13, 0.13] |
| 1.3 Headache relief | 2 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.11, 2.00] |
| 1.4 Rescue medication | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.02, 1.56] |
| 1.5 Headache recurrence | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.01, 5.68] |
Comparison 2. Triptans vs placebo in children.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Pain‐free | 3 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [1.06, 2.62] |
| 2.1.1 Rizatriptan | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.89, 1.92] |
| 2.1.2 Sumatriptan | 2 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 2.29 [1.00, 5.23] |
| 2.2 Adverse events (any) | 3 | 420 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [‐0.04, 0.17] |
| 2.2.1 Rizatriptan | 1 | 275 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.09, 0.09] |
| 2.2.2 Sumatriptan | 2 | 145 | Risk Difference (M‐H, Random, 95% CI) | 0.13 [0.01, 0.26] |
| 2.3 Headache relief | 3 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.88, 2.08] |
| 2.3.1 Rizatriptan | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.75, 1.24] |
| 2.3.2 Sumatriptan | 2 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [1.21, 2.26] |
| 2.4 Rescue medication | 2 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.06, 1.45] |
| 2.4.1 Sumatriptan | 2 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.06, 1.45] |
| 2.5 Headache recurrence | 1 | 18 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 2.5.1 Sumatriptan | 1 | 18 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 2.6 Presence of nausea | 3 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.46, 0.90] |
| 2.6.1 Rizatriptan | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.38, 1.22] |
| 2.6.2 Sumatriptan | 2 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.42, 0.94] |
| 2.7 Presence of vomiting | 3 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.13, 1.86] |
| 2.7.1 Rizatriptan | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [0.43, 7.06] |
| 2.7.2 Sumatriptan | 2 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.09, 0.99] |
Comparison 3. Triptans vs placebo in adolescents.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Pain‐free | 21 | 6761 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [1.19, 1.47] |
| 3.1.1 Almotriptan | 1 | 714 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.88, 1.39] |
| 3.1.2 Eletriptan | 1 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.88, 2.43] |
| 3.1.3 Naratriptan | 1 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.65, 1.75] |
| 3.1.4 Rizatriptan | 4 | 1526 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [1.13, 1.60] |
| 3.1.5 Sumatriptan | 10 | 2415 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.10, 1.48] |
| 3.1.6 Zolmitriptan | 4 | 1532 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.16, 2.38] |
| 3.2 Adverse events (any) | 21 | 7876 | Risk Difference (M‐H, Random, 95% CI) | 0.13 [0.08, 0.18] |
| 3.2.1 Almotriptan | 1 | 720 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [0.00, 0.09] |
| 3.2.2 Eletriptan | 1 | 242 | Risk Difference (M‐H, Random, 95% CI) | 0.14 [0.02, 0.26] |
| 3.2.3 Naratriptan | 1 | 300 | Risk Difference (M‐H, Random, 95% CI) | 0.16 [0.05, 0.27] |
| 3.2.4 Rizatriptan | 4 | 1706 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.02, 0.10] |
| 3.2.5 Sumatriptan | 10 | 2969 | Risk Difference (M‐H, Random, 95% CI) | 0.18 [0.09, 0.27] |
| 3.2.6 Zolmitriptan | 4 | 1939 | Risk Difference (M‐H, Random, 95% CI) | 0.14 [0.07, 0.20] |
| 3.3 Headache relief | 20 | 6182 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [1.04, 1.24] |
| 3.3.1 Almotriptan | 1 | 714 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.10, 1.47] |
| 3.3.2 Eletriptan | 1 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.79, 1.17] |
| 3.3.3 Naratriptan | 1 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.65, 1.00] |
| 3.3.4 Rizatriptan | 4 | 1526 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.99, 1.56] |
| 3.3.5 Sumatriptan | 10 | 2392 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [1.02, 1.28] |
| 3.3.6 Zolmitriptan | 3 | 973 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.87, 1.69] |
| 3.4 Rescue medication | 18 | 5066 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.72, 0.87] |
| 3.4.1 Almotriptan | 1 | 714 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.28, 1.16] |
| 3.4.2 Eletriptan | 1 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.59, 1.12] |
| 3.4.3 Naratriptan | 1 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.55, 1.53] |
| 3.4.4 Rizatriptan | 3 | 956 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.55, 1.01] |
| 3.4.5 Sumatriptan | 10 | 2451 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.70, 0.92] |
| 3.4.6 Zolmitriptan | 2 | 368 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.20, 1.52] |
| 3.5 Headache recurrence | 15 | 2463 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.68, 0.93] |
| 3.5.1 Almotriptan | 1 | 477 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.44, 2.89] |
| 3.5.2 Eletriptan | 1 | 161 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.53, 1.23] |
| 3.5.3 Naratriptan | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.42, 1.67] |
| 3.5.4 Rizatriptan | 1 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.30, 1.32] |
| 3.5.5 Sumatriptan | 9 | 1319 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.65, 0.94] |
| 3.5.6 Zolmitriptan | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.36, 2.65] |
| 3.6 Presence of nausea | 17 | 4975 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.79, 1.12] |
| 3.6.1 Almotriptan | 1 | 356 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.04, 2.02] |
| 3.6.2 Eletriptan | 1 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.84, 1.09] |
| 3.6.3 Naratriptan | 1 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.66, 1.78] |
| 3.6.4 Rizatriptan | 2 | 1060 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.49, 0.80] |
| 3.6.5 Sumatriptan | 10 | 2279 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.78, 1.29] |
| 3.6.6 Zolmitriptan | 2 | 703 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.04, 4.02] |
| 3.7 Presence of vomiting | 12 | 4037 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.48, 1.12] |
| 3.7.1 Almotriptan | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 3.7.2 Eletriptan | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 3.7.3 Naratriptan | 1 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [0.24, 16.05] |
| 3.7.4 Rizatriptan | 1 | 769 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.22, 1.18] |
| 3.7.5 Sumatriptan | 8 | 2265 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.39, 1.20] |
| 3.7.6 Zolmitriptan | 2 | 703 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.79, 1.58] |
Comparison 4. Triptans vs placebo in adolescents, subgroup analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Pain‐free by route (oral or intranasal) | 21 | 6764 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [1.19, 1.47] |
| 4.1.1 Oral | 15 | 4415 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.08, 1.38] |
| 4.1.2 Intranasal | 6 | 2349 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [1.32, 1.75] |
| 4.2 Sumatriptan vs placebo by route (oral or intranasal) | 10 | 2415 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.10, 1.48] |
| 4.2.1 Intranasal | 4 | 1490 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [1.15, 1.63] |
| 4.2.2 Oral | 6 | 925 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.75, 1.43] |
| 4.3 Zolmitriptan vs placebo by route (oral or intranasal) | 4 | 1532 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.16, 2.38] |
| 4.3.1 Intranasal | 2 | 859 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [1.46, 2.40] |
| 4.3.2 Oral | 2 | 673 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [0.42, 9.07] |
| 4.4 Pain‐free by preventive medication | 21 | 6764 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [1.19, 1.47] |
| 4.4.1 Preventive medication not permitted | 8 | 1658 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.14, 1.69] |
| 4.4.2 Preventive medication permitted | 8 | 2316 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.04, 1.56] |
| 4.4.3 Unsure | 5 | 2790 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.11, 1.61] |
Comparison 5. Triptans vs placebo by age, subgroup analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Age group | 23 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1.1 Children | 3 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [1.06, 2.62] |
| 5.1.2 Mixed children and adolescents | 5 | 606 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [0.96, 2.98] |
| 5.1.3 Adolescent | 16 | 6188 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.18, 1.45] |
Comparison 6. Triptans vs placebo in adolescents, sensitivity analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Study design | 21 | 6794 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.19, 1.49] |
| 6.1.1 Cross‐over | 7 | 1127 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [1.44, 2.26] |
| 6.1.2 Parallel | 14 | 5667 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.12, 1.39] |
| 6.2 Allocation concealment | 21 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.2.1 Low | 8 | 2365 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [1.19, 1.89] |
| 6.2.2 Unclear | 13 | 4429 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.12, 1.40] |
| 6.3 Source of funding | 21 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.3.1 Pharmaceutical | 17 | 6332 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.16, 1.43] |
| 6.3.2 Non‐pharmaceutical | 2 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 4.22 [1.50, 11.84] |
| 6.3.3 Unclear | 2 | 358 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [1.22, 2.54] |
| 6.4 Reported in a journal | 21 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.4.1 Journal report | 15 | 5175 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [1.18, 1.52] |
| 6.4.2 Registry or abstract only | 6 | 1619 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.94, 1.71] |
| 6.5 Sample size | 21 | 6794 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.19, 1.49] |
| 6.5.1 Sample size ≤ 50 | 2 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 4.22 [1.50, 11.84] |
| 6.5.2 Sample size > 50 | 19 | 6690 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.18, 1.46] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahonen 2004.
| Study characteristics | ||
| Methods | Randomized, double‐blind, placebo‐controlled, two‐way cross‐over trial of sumatriptan nasal spray | |
| Participants | Children and adolescents 8‐17 years and body weight ≥ 20 kg with a diagnosis of migraine with or without aura meeting the IHS 1988 criteria from 3 pediatric hospitals in Finland. Participants were expected to have least 2 migraine attacks per month lasting 4 h and demonstrated resistance to usual therapy for migraine with paracetamol or nonsteroidal anti‐inflammatory drugs (NSAIDs). No participants were receiving preventive medication. Randomized (N = 129); medication not used (N = 35); 1 medication used (N = 11); intention‐to‐treat analysis (N = 94); primary efficacy analysis (N = 83) |
|
| Interventions | Sumatriptan nasal spray 10 mg (weight 20 to 39 kg) or 20 mg (≥ 40 kg) versus placebo. Children were instructed to take a single nasal insufflation at the onset of a migraine attack if headache severity was classified as ≥ 3 on a 5‐faces pain intensity scale. Each participant treated 2 migraine attacks ‐ 1 with placebo and the other with sumatriptan. Rescue medications were allowed at any point. | |
| Outcomes |
Other reported outcomes:
|
|
| Headache severity scale | 5‐faces pain scale (5 severe, 4 to 3 moderate, 2 mild, 1 no pain) | |
| Funding source | GlaxoSmithKline | |
| Publication | Journal | |
| Notes | All children who had used at least 1 treatment were included in the intention‐to‐treat analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done in the hospital pharmacy" |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization code was stored by one of the authors who did not meet any of the patients, and it was not broken until the database of treatment efficacy was created and locked" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "study drugs of identical appearance"; "indistinguishable". Comment: Probably done, since both preparations were supplied by GlaxoSmithKline |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: study drug and placebo groups were balanced |
| Selective reporting (reporting bias) | Low risk | Comment: all primary outcomes were reported |
Ahonen 2006.
| Study characteristics | ||
| Methods | Randomized, placebo‐controlled, double‐blind, 3‐way cross‐over trial of oral rizatriptan | |
| Participants | Children or adolescents between 6 and 17 years of age, body weight ≥ 20 kg, headache meeting the IHS 1988 criteria for migraine with or without aura from the 2 pediatric hospitals in Finland. Participants were expected to have at least 2 migraine attacks per month lasting 4 h or more and previous unsatisfactory with paracetamol or NSAIDs. None of the children were receiving preventive therapy. Randomized (N = 147); medication not used (N = 31); 1 medication used (N = 10); 2 treatments used (N = 10); intention‐to‐treat analysis (N = 116); primary efficacy analysis (N = 96) |
|
| Interventions | Rizatriptan 5 mg (weight 20 to 39 kg) or rizatriptan 10 mg (weight ≥ 40 kg) and placebo. Each participant treated 3 migraine attacks (2 with rizatriptan and 1 with placebo) at the onset of the attack if headache severity was ≥ 3 on the 5‐point scale. Rescue medications were allowed at any time, but encouraged to be taken only after 2 h. | |
| Outcomes |
Other reported outcomes:
|
|
| Headache severity scale | 5‐faces pain scale (5 severe, 4 to 3 moderate, 2 mild, 1 no pain) | |
| Funding source | Not specified | |
| Publication | Journal | |
| Notes | All children who had used at least 1 treatment were included in the intention‐to‐treat analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "tossing a coin method" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was done in the hospital pharmacy"; "randomization code was stored by one of the authors. . . was not broken until the database of treatment efficacy was created and locked" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "drugs packed in capsules of identical appearance" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: missing outcome data balanced across intervention groups |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
Callenbach 2007.
| Study characteristics | ||
| Methods | Randomized, double‐blind, placebo‐controlled, cross‐over, 2‐attack study of sumatriptan nasal spray | |
| Participants | Participants from 18 centres in the Netherlands were 12‐17 years of age with a diagnosis of migraine with or without aura per revised IHS 1988 criteria (Winner 1995) whom had failed, or responded inadequately, to at least 1 over the counter or prescription medication for migraine, duration typically longer than 4 hours if untreated, and between 1 and 8 attacks per month in each of the 2 months before enrolment Randomized (N = 66); withdrawn (N = 20); analyzed (N = 46) |
|
| Interventions | Each participant treated 2 attacks ‐ 1 with sumatriptan nasal spray (10 mg if < 40 kg or 20 mg if > 40 kg) and 1 with placebo nasal spray. Recurrence within 2‐24 h could be treated with a second dose. | |
| Outcomes |
Other reported outcomes:
|
|
| Headache severity scale | 4‐point scale (none, mild, moderate, severe) | |
| Funding source | GlaxoSmithKline | |
| Publication | Journal and Clinical Trial Registry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "in a randomized order" Comment: other studies funded by GlaxoSmithKline reported acceptable sequence generation (e.g. Winner 2007) |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: withdrawals balanced across intervention groups |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
Derosier 2012.
| Study characteristics | ||
| Methods | Outpatient, double‐blind, randomized, placebo‐controlled, parallel group trial with an enrichment design of oral sumatriptan + naproxen sodium | |
| Participants | Eligible participants were 12 to 17 years old at screening and had > 6 months history of 2 to 8 migraine attacks per month (with or without aura: ICHD‐2), typically lasting > 3 h and associated with moderate to severe headache pain. Triptan‐naïve subjects were eligible. Run‐in phase (N = 976); Randomized (N = 589); Medication not used (N = 57); Withdrawn (N = 42); intention‐to‐treat and primary efficacy analysis (N = 490) |
|
| Interventions | Subjects treating 1 moderate to severe migraine with single‐blind placebo during the run‐in phase and reporting pain 2 h post dose (placebo non responders) were randomly assigned into the double‐blind phase. In the double‐blind phase, subjects treated 1 moderate to severe migraine with either matching placebo or sumatriptan + naproxen sodium 10 mg + 60 mg, 30 mg + 180 mg, or 85 mg + 500 mg. | |
| Outcomes |
Other reported outcomes:
|
|
| Headache severity scale | 4‐level pain scale (none, mild, moderate, severe) | |
| Funding source | GlaxoSmithKline | |
| Publication | Journal and Clinical Trial Registry | |
| Notes | Single‐blind run‐in phase (enrichment design) assessment was at 2 h | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Upon determination of subject eligibility and completion of the randomizations visit, site staff telephoned an interactive voice response system and dispensed randomly assigned, age‐group stratified treatment." |
| Allocation concealment (selection bias) | Low risk | Comment: used interactive voice response system for randomization |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Parent/guardian and subject were informed that they would not know which study drug was provided for the first or second migraine treated, and that the investigator would not know which study drug was provided for the second migraine treated. To maintain blinding, sites received education on describing the single‐blind phase. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: withdrawals and reasons for withdrawal (outlined in Figure 1 of Derosier 2012) were balanced between groups |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes were reported |
Evers 2006.
| Study characteristics | ||
| Methods | Double‐blind, placebo‐controlled, cross‐over study to investigate the efficacy of oral zolmitriptan in the treatment of migraine attacks in children and adolescents | |
| Participants | Children and adolescents aged 6‐18 years from an headache outpatient clinic (not reported separately)
Migraine with and without aura according to the criteria of the ICHD‐2
No neurologic, psychiatric, and vascular disorder Randomized (N = 32); withdrawn (N = 3); intention‐to‐treat and primary efficacy analysis (N = 29) |
|
| Interventions | Ibuprofen 200 mg PO (children under 12) or 400 mg PO (adolescents) Zolmitriptan 2.5 mg PO |
|
| Outcomes |
Other reported outcomes:
|
|
| Headache severity scale | 4‐point scale (none, mild, moderate, severe) | |
| Funding source | Not clear | |
| Publication | Journal | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The order of the study drugs was randomized for every patient." |
| Allocation concealment (selection bias) | Low risk | Comment: cross‐over study where each participant received the study drugs in random order |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: blinding was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 3 of 32 participants dropped out (withdrawal of consent N = 2) lost to follow‐up N = 1) |
| Selective reporting (reporting bias) | Low risk | Comment: aAll primary and secondary outcomes were reported |
Fujita 2014.
| Study characteristics | ||
| Methods | Randomized, double‐blind, placebo‐controlled, parallel group trial of oral sumatriptan | |
| Participants | Participants from 17 centres in Japan were 10‐17 years of age, diagnosed with migraine with or without aura per ICHD‐2 criteria for a minimum of 6 months, and 2‐8 attacks monthly lasting for > 3 h in the last 2 months prior to entry. Randomized (N = 178); withdrawn (N = 34); intention‐to‐treat and primary efficacy analysis (N = 144) |
|
| Interventions | Oral sumatriptan 25 mg (1 tablet and 1 matching placebo), sumatriptan 50 mg (2 tablets), or placebo (2 tablets) taken as soon as possible (within 30 minutes) after the development of a migraine with grade 3 or more pain | |
| Outcomes |
Other reported outcomes:
|
|
| Headache severity scale | 5‐grade scale | |
| Funding source | GlaxoSmithKline | |
| Publication | Journal and Clinical Trial Registry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Investigator (or subinvestigator) completed the registration form and sent it to the randomization center by facsimile. The randomization center. . . assigned a randomization number to the patient." |
| Allocation concealment (selection bias) | Low risk | Quote: "For allocation of the participants, a computer‐generated list of random numbers was used." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The investigator (or subinvestigator) dispensed the investigational product to the patient according to the computer‐generated randomization number." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: used last observation carried forward (LOCF) |
| Selective reporting (reporting bias) | Low risk | Comment: all stated outcomes were reported |
Ho 2012.
| Study characteristics | ||
| Methods | Randomized, double‐blind, placebo‐controlled, parallel group trial of oral rizatriptan with an enrichment design | |
| Participants | Participants were males and females aged 6–17 years who were ≥ 20kg in weight and who had at least a 6‐month history of migraine attacks with or without aura as defined by ICHD‐2, usually lasting 3 h or more (when untreated). Participants had ≥ 1 and ≤ 8 moderate to severe migraine attacks with or without aura per month in the 2 months before the screening visit, and had not, by history, experienced satisfactory relief with NSAIDs or paracetamol. Patients were excluded if they had not experienced satisfactory relief from migraine pain during prior treatment with 2 or more adequate courses of triptans. Non‐responders to placebo in stage 1 were randomized 1:1 to rizatriptan:placebo, with randomization stratified by age (6–11 years versus 12–17 years). Randomized (N = 1382); not treated (N = 405); randomized in stage 1 (N = 915); randomized in stage 2 (N = 791); withdrawn (N = 21); intention‐to‐treat and primary efficacy analysis (N = 770) |
|
| Interventions | Oral‐disintegrating tablet of rizatriptan 5 mg (< 40 kg) or 10 mg (≥ 40 kg) or placebo. Participants treated within 30 min of a moderate/severe migraine attack. In stage 1, participants were randomized 20:1 to placebo or rizatriptan. In stage 2, participants with ongoing moderate/severe pain after 15 min (non‐responders) who received placebo in stage 1 were randomized 1:1 to rizatriptan or placebo. | |
| Outcomes |
Other reported outcomes:
|
|
| Headache severity scale | 4‐point scale (none, mild, moderate, severe) | |
| Funding source | Merck | |
| Publication | Journal and abstract | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomization process not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation performed using interactive voice response system |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: withdrawals are balanced between intervention groups |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported including adverse events |
Hämäläinen 1997a.
| Study characteristics | ||
| Methods | Randomized, double‐blind, placebo‐controlled, 3‐way cross‐over trial of ibuprofen, paracetamol, and placebo | |
| Participants | Children or adolescents < 18 years with a diagnosis of migraine with or without aura meeting IHS 1988 criteria from 3 pediatric hospitals in the Greater Helsinki Area of Finland who found previous therapy for migraine unsatisfactory. Participants were required to have 2 migraine attacks per month lasting 2 h or more. Randomized (N = 106); lost to follow‐up (N = 2); medication not used (N = 16); 1 medication used (N = 5); 2 medications used (N = 8); withdrawn (N = 9); intention‐to‐treat analysis (N = 88); primary efficacy analysis (N = 66) |
|
| Interventions | Each participant treated 1 of 3 migraine attacks with either oral paracetamol (15 mg/kg), oral ibuprofen (10 mg/kg), or placebo. The active drugs and matching placebo were supplied by the University Pharmacy of Helsinki in 3 mixtures containing peppermint water, black currant syrup, sugar syrup, and either 30 mg/ml paracetamol or 20 mg/ml ibuprofen, or, as a placebo, cellulose. Each participant received a package of 3 identically numbered bottles and a plastic 10 ml syringe for exact weight‐based dosing (0.5 ml/kg, maximum dose 30 ml). | |
| Outcomes |
Other reported outcomes:
|
|
| Headache severity scale | Participants were allowed to choose between the 5‐faces pain scale (5 severe, 4 to 3 moderate, 2 mild, 1 no pain) or the 100 mm visual analogue scale (VAS). The VAS (0 to 100) data were transformed to a nominal scale: grade 1: 0 to ≤ 12; grade 2: 12 to ≤ 37; grade 3: 37 to ≤ 62; grade 4: 62 to ≤ 87; and grade 5: 87 to ≤ 100 | |
| Funding source | Not specified | |
| Publication | Journal | |
| Notes | All additional children and adolescents with any data on efficacy were included in the intention‐to‐treat analysis, which was performed without regard to pain intensity at the start of the attack. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "[T]he active drugs and matching placebo were supplied by the University Pharmacy of Helsinki in three mixtures containing peppermint water, black currant syrup, sugar syrup, and either 30 mg/ml paracetamol or 20 mg/ml ibuprofen, or as placebo (cellulose). Each participant received a package of three identically numbered bottles and a plastic 10 ml syringe for exact weight‐based dosing (0.5 ml/kg; max 30 ml)." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: missing outcome data balanced across intervention groups |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
Hämäläinen 1997b.
| Study characteristics | ||
| Methods | Randomized, double‐blind, placebo‐controlled, 2‐way cross‐over trial of oral sumatriptan | |
| Participants | Children or adolescents 8‐17 years with a diagnosis of migraine with or without aura meeting IHS 1988 criteria from 3 pediatric hospitals in the Greater Helsinki Area of Finland who had not benefited from previous migraine therapy. Participants were required to have 2 migraine attacks per month. None of the participants were using preventive therapy, but had already participated in previous placebo‐controlled trials with paracetamol and ibuprofen, dihydroergotamine, or both. Randomized (N = 31); medication not used (N = 4); 1 medication used (N = 3); withdrawn (N = 1); primary efficacy analysis (N = 23) |
|
| Interventions | Each participant treated 1 migraine attack at home with oral sumatriptan (50 mg for a body surface area of 0.75‐1.5 m2 (~6‐12 years of age) and 100 mg for a body surface area greater than 1.5 m2 (~>12 years of age) and 1 migraine attack with placebo in a randomized order. Rescue medications were permitted at any time. | |
| Outcomes |
Other reported outcomes:
|
|
| Headache severity scale | 100 mm VAS | |
| Funding source | Arvo, Lea Ylppo Foundation, and Academy of Finland | |
| Publication | Journal | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "matching placebo. . . Each patient received two identical packages" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: missing outcome data balanced across intervention groups |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes were reported |
Hämäläinen 1997c.
| Study characteristics | ||
| Methods | Randomized, double‐blind, placebo‐controlled, 4‐way cross‐over trial of oral dihydroergotamine | |
| Participants | Children or adolescents < 18 years with a diagnosis of migraine per IHS 1988 criteria and at least 2 migraine attacks per month. Most participants had participated in a previous trial of paracetamol and ibuprofen versus placebo. Randomized (N = 16); 1 medication used (N = 13); analyzed (N = 12) |
|
| Interventions | Dihydroergotamine (DHE) mesylate oral solution 2 mg/ml and placebo. Each participant treated 1 migraine attack at home with DHE 20 µg/kg (DHE solution 2 drops/10 kg) and 1 with placebo. If the first dose provided any relief, a second dose could be taken after 1 h. If neither treatment produced an adequate response, 1 further migraine attack was treated with DHE 40 µg/kg and 1 with placebo after contact with the investigator. | |
| Outcomes |
Other reported outcomes:
|
|
| Headache severity scale | 5‐point scale (5 severe, 3‐4 moderate, 2 mild, 1 no pain) | |
| Funding source | Not specified | |
| Publication | Journal | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "each patient received two identical bottles" Comment: the taste may have been different between the bottles |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: patients who were excluded were not described |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
Hämäläinen 2002.
| Study characteristics | ||
| Methods | Randomized, double‐blind, placebo‐controlled, single attack cross‐over trial of sumatriptan nasal spray | |
| Participants | Participants from 1 center in Germany were children 8‐12 years of age with 6‐month history of migraine with or without aura per IHS 1988 criteria, an attack frequency of 2‐8 monthly, and minimum duration of 4 h. Randomized (N = 60); withdrawn (N = 3); intention‐to‐treat analysis and primary efficacy analysis (N = 59) |
|
| Interventions | Each participant treated 1 migraine attack with nasal sumatriptan (10 mg) or placebo nasal spray. The second migraine was treated with the other medication | |
| Outcomes |
Other reported outcomes:
|
|
| Headache severity scale | 4‐point scale (none, mild, moderate, severe) | |
| Funding source | GlaxoSmithKline | |
| Publication | Clinical trial registry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized at visit one". Comment: probably done as with previous studies by sponsor but not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomized at visit one and received a single dose of sumatriptan 10 mg nasal spray or placebo to treat one migraine attack. At visit 2 they received the alternate treatment. . ." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: missing outcome data balanced across intervention groups |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
Lewis 2002.
| Study characteristics | ||
| Methods | Randomized, double‐blind, placebo‐controlled, parallel‐group trial of oral ibuprofen | |
| Participants | Participants were 6‐12 years of age and met diagnostic criteria for migraine without aura per revised IHS 1988 criteria for children (Winner 1995) from multiple sites in the United States. Enrolled (N = 138); treated/completed diary (N = 84) |
|
| Interventions | Each participant treated 1 migraine with liquid ibuprofen suspension (7.5 mg/kg) or placebo | |
| Outcomes |
Other reported outcomes:
|
|
| Headache severity scale | 4‐point scale (none, mild, moderate, severe) | |
| Funding source | Not specified | |
| Publication | Journal | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomly assigned (stratified by gender) to the study medication in a double‐blind fashion." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Subjects were randomized to one of the following groups in a 1:1 ratio" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "matching placebo suspension" Comment: no description of taste or color |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Fifty‐four patients were randomized but were not evaluable. . . Six treated with study agent" Comment: missing outcome data balanced between intervention groups |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes were reported |
Lewis 2007.
| Study characteristics | ||
| Methods | Multicenter, randomized, double‐blind, placebo‐controlled, 2‐way, 2‐attack, cross‐over study of zolmitriptan nasal spray with a single‐blind 'placebo challenge' or 'enrichment' phase | |
| Participants | Adolescents of age 12‐17 years with a diagnosis of migraine with or without aura per the IHS 1988 criteria or revised IHS‐1988 criteria (Winner 1995) with a frequency of 2 or more migraine attacks per month during the school year and < 14 days per month without migraine for the 3 months before screening. The usual duration of untreated migraine was required to be > 2 h. Participants were permitted to use preventive medication that had been stable for 2 months before randomization. Randomized (N = 248); withdrawn (N = 34); placebo responder to both attacks (N = 12); placebo responder to 1 attack (N = 12); missing efficiency data (N = 19); intention‐to‐treat analysis (N = 171) |
|
| Interventions | Each participant treated 1 migraine attack with zolmitriptan 5 mg nasal spray and another with matching placebo within a 12‐week period. Each attack was treated initially with placebo normal saline within 30 min after the headache reached moderate or severe intensity. If migraine intensity remained moderate or severe, then participants were randomly assigned to use zolmitriptan or matching placebo (1:1). A second dose of randomized treatment or approved escape medications were permitted 2 h after the first dose if moderate or severe pain persisted. | |
| Outcomes |
Other reported outcomes:
|
|
| Headache severity scale | 4‐point scale (none, mild, moderate, severe) | |
| Funding source | AstraZeneca | |
| Publication | Journal and clinical trial registry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned strictly sequentially" |
| Allocation concealment (selection bias) | Low risk | Quote: "double‐blind randomization schedule (prepared by the study sponsor)" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "matching placebo" Comment: unique study design with placebo challenge where all subjects initially received placebo in a single‐blind (participant) fashion. Only those who did not respond were randomly assigned to active drug or placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: missing outcome data distributed evenly across intervention groups |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
Linder 2008.
| Study characteristics | ||
| Methods | Randomized, double‐blind, placebo‐controlled, parallel‐group multicenter trial of oral almotriptan | |
| Participants | Participants from the United States (81 sites), Argentina (6 sites), Colombia (3 sites), and Mexico (3 sites) diagnosed with migraine were aged 12‐17 years (block randomization in 2 groups: 12‐14 years and 15‐17 years). The participants were required to have a > 1‐year history of migraine with or without aura per the IHS 1988 criteria and a frequency of 1‐6 moderate or severe attacks per month, lasting > 4 h without treatment and occurring at intervals of > 24 h for the 2 months prior to screening. Following a 30‐day run‐in period to document migraine severity and frequency, eligible participants were randomized 1:1:1:1 within the 2 age groups to treat 1 attack. Enrolled (N = 866); did not receive medication (N = 146); non‐compliant (N = 6); safety analysis (N = 720); primary efficacy analysis (N = 714) |
|
| Interventions | Each participant treated 1 attack with either oral almotriptan (6.25 mg, 12.5 mg, or 25 mg) or placebo as soon as possible within 4 h after the onset of moderate to severe pain. | |
| Outcomes |
Other reported outcomes:
|
|
| Headache severity scale | 4‐point scale (none, mild, moderate, severe) | |
| Funding source | Janssen‐Ortho LLC: Ortho‐McNeil Neurologics, Inc. | |
| Publication | Journal and clinical trial registry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized 1:1:1:1 within 2 age groups" |
| Allocation concealment (selection bias) | Unclear risk | Comment: each participant received 2 tablets of either study medication or placebo |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: described as 'double‐blind'. Previous studies by sponsor included acceptable blinding methods (e.g. Lewis 2007). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: missing outcome data balanced across intervention groups |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
NCT01211145.
| Study characteristics | ||
| Methods | Randomized, double‐blind, placebo‐controlled, parallel group trial of zolmitriptan nasal spray | |
| Participants | Participants from 74 study sites worldwide were adolescents 12‐17 years of age, diagnosed with migraine per IHS 1988 or revised IHS criteria (Winner 1995) and a history of at least 2 migraine attacks per month. Lack of response to single‐blind placebo run‐in period was also required. Randomized (N = 798); did not receive study drug (N = 141); analyzed (N = 584) |
|
| Interventions | Zolmitriptan 0.5, 2.5, 5 mg nasal spray | |
| Outcomes |
Other reported outcomes:
|
|
| Headache severity scale | 4‐point scale (none, mild, moderate, severe) | |
| Funding source | AstraZeneca | |
| Publication | Clinical trial registry | |
| Notes | At the interim futility analysis, the zolmitriptan 0.5 and 2.5 mg groups were declared futile, allocation discontinued, and not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "were randomized into the study to obtain 800 evaluable patients" Comment: no description of randomization procedures provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: study is described as blinded but no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of the 798 patients, 82.3% (657/798 patients) received study drug, 90.4% (721/798 patients) completed the study and 9.5% (76/798 patients) discontinued from the study. . . All patients received their assigned treatment. . . Overall, the most common reason for study discontinuation was eligibility criteria not fulfilled (6.6%, 53/798 patients). No patients discontinued due to AEs." |
| Selective reporting (reporting bias) | Unclear risk | Comment: a clinical study report synopsis was reported with limited data |
Rothner 1997.
| Study characteristics | ||
| Methods | Randomized, double‐blind, placebo‐controlled, parallel trial of oral naratriptan | |
| Participants | Participants were from the United States (44 sites), aged 12‐17 years with a 1‐year history of migraine with or without aura as per the IHS 1988 criteria and had 1‐8 migraine attacks per month of moderate to severe intensity during the 2 months preceding the screening visit. Randomized (N = 350); withdrawn (N = 51); intention‐to‐treat and primary efficacy analysis (N = 300) |
|
| Interventions | Each participant treated 1 migraine with oral naratriptan (0.25 mg, 1.0 mg, or 2.5 mg) or placebo. A second, identical dose was available (optional) for participants who experienced recurrence. | |
| Outcomes |
Other reported outcomes:
|
|
| Headache severity scale | 4‐point scale (none, mild, moderate, severe) | |
| Funding source | GlaxoSmithKline | |
| Publication | Abstract and clinical trial registry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomized (1:1:1:1)" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: described as double‐blind and previous studies by sponsor had acceptable blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: missing outcome data balanced across intervention groups |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
Rothner 1999a.
| Study characteristics | ||
| Methods | Randomized, double‐blind, placebo‐controlled, parallel‐group 3‐attack trial of oral sumatriptan | |
| Participants | Participants from 62 centers in 7 countries were 12‐17 years of age with a minimum 6‐month history of migraine with or without aura per IHS 1988 criteria and 1‐6 attacks per month Randomized (N = 347); withdrawn (N = 117); intention‐to‐treat and primary efficacy analysis (N = 273) |
|
| Interventions | Each participant treated 1 migraine with oral sumatriptan 25 mg, 50 mg, 100 mg, or placebo. No maximum time after the onset of migraine was specified. | |
| Outcomes |
Other reported outcomes:
|
|
| Headache severity scale | 4‐point scale (none, mild, moderate, severe) | |
| Funding source | GlaxoSmithKline | |
| Publication | Abstract and clinical trial registry | |
| Notes | Headache alleviation: reduction in headache from grade 3 or 2 to grade 1 or 0 within 2 h where 3 = headache, I can't do anything; 2 = headache, I can do easy activities; 1 = headache, I can carry on as usual; and 0 = no headache. Funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: described as randomized but no details |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: no description of efforts to maintain blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: missing outcome data balanced across intervention groups |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
Rothner 1999b.
| Study characteristics | ||
| Methods | Randomized, double‐blind, placebo‐controlled, parallel‐group trial of oral sumatriptan | |
| Participants | Participants from Canada (14 sites) were aged 12‐17 years, had a 3‐month history of migraine with or without aura as per the IHS 1988 criteria (except headaches could be < 2 h) and the migraine attack had lasted < 24 h. Randomized (N = 119); withdrawn (N = 27); intention‐to‐treat and primary efficacy analysis (N = 92) |
|
| Interventions | Each participant treated 1 migraine with oral sumatriptan (50 mg for body weight 30‐50kg; 100 mg for body weight > 50kg) or placebo. Use of rescue medication was permitted after 4 h of study treatment. | |
| Outcomes |
Other reported outcomes:
|
|
| Headache severity scale | 4‐point scale (none, mild, moderate, severe) | |
| Funding source | GlaxoSmithKline | |
| Publication | Abstract and clinical trial registry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomized (1:1:1 ratio) to receive. . ." |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: described as "placebo‐controlled"; participant‐reported outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: missing outcome data balanced across intervention groups. |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
Rothner 1999c.
| Study characteristics | ||
| Methods | Randomized, double‐blind, placebo‐controlled, parallel‐group trial of oral sumatriptan | |
| Participants | Participants were from 18 centers in 8 countries and 12‐17 years of age with migraine with or without aura per IHS 1988 criteria; treated 1 attack following a run‐in period of up to 8 weeks during which their usual medication was used to treat 1 migraine attack Randomized (N = 139); withdrawn (N = 37); intention‐to‐treat and primary efficacy analysis (N = 102) |
|
| Interventions | Oral sumatriptan 50 mg (30 to 50 kg) or sumatriptan 100 mg (> 50 kg) versus placebo | |
| Outcomes |
Other reported outcomes:
|
|
| Headache severity scale | Headache/clinical disability score (grade 3 = headache ‐ "I can’t do anything"; grade 2 = headache – "I can do easy activities"; grade 1 = headache – "I can carry on as usual", grade 0 = no headache) | |
| Funding source | GlaxoSmithKline | |
| Publication | Abstract and clinical trial registry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: described as "randomized in a 1:1:1 ratio. . ." |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of methods to maintain allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: described as "double‐blind"; previous studies by sponsor had adequate blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: withdrawals balances between intervention groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: outcomes extensively reported in the GlaxoSmithKline clinical trial registry |
Rothner 2006.
| Study characteristics | ||
| Methods | Randomized, double‐blind, placebo‐controlled, parallel‐group trial of oral zolmitriptan | |
| Participants | Participants were from the United States (40 sites), Canada (10 sites), India (23 sites), Finland, Germany, and the United Kingdom. Eligible participants were aged 12‐17 years with a diagnosis of migraine with or without aura per the IHS 1988 criteria and were required to have 2‐10 migraine or non‐migraine headaches per month lasting longer than 4 h without treatment in the last 3 months preceding screening Randomized (N = 850); did not treat at least 1 attack (N = 151); intention‐to‐treat and primary efficacy analysis (N = 696) |
|
| Interventions | Each participant treated 1 migraine attack with oral zolmitriptan (2.5 mg, 5 mg, or 10 mg) or placebo no later than 1 h after the onset of moderate or severe headache, more than 24 h had elapsed since the last migraine attack and the migraine attack occurred within 12 weeks of randomization. Participants were allowed to take an approved escape medication 2 or more h after study treatment. | |
| Outcomes |
Other reported outcomes:
|
|
| Headache severity scale | 4‐point scale (none, mild, moderate, severe) | |
| Funding source | AstraZeneca | |
| Publication | Journal and clinical trial registry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "numbers allocated sequentially as patients entered the study" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The randomization schedule was produced by the Biometrics Group of AstraZeneca." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "three tablets, all of which were identical in appearance" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: missing outcome data balanced across intervention groups |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
Ueberall 1999.
| Study characteristics | ||
| Methods | Randomized, double‐blind, placebo‐controlled, 2‐way cross‐over trial of intranasal sumatriptan | |
| Participants | Participants were < 10 years of age with migraine with or without aura per IHS 1988 criteria and at least 2 migraine attacks per month Randomized (N = 14); analyzed (N = 14) |
|
| Interventions | Each participant treated 1 migraine with intranasal sumatriptan (20 mg) or placebo. The second migraine was treated with the other medication. | |
| Outcomes |
Other reported outcomes:
|
|
| Headache severity scale | 4‐point scale (none, mild, moderate, severe) | |
| Funding source | Not specified | |
| Publication | Journal | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was based on a computer algorithm. . ." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to two alternative treatment strata"; "access to treatment assignments were given to D.W., if necessary" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "0.9% sodium chloride" used as placebo Comment: cross‐over trial; blinding likely maintained. Disturbance of taste reported both for sumatriptan and placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Data sufficient for analysis were provided by all 14 children". |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported |
Visser 2004a.
| Study characteristics | ||
| Methods | Randomized, double‐blind, placebo‐controlled, parallel‐group single‐attack trial of oral rizatriptan | |
| Participants | Participants from 44 centers in the United States 12‐17 years of age with a 1 year history of migraine with or without aura per IHS 1988 criteria and 1‐8 attacks per month. Randomization was stratified by age (12‐14 and 15‐17 years). Randomized (N = 686); did not take study medication (N = 210); primary efficacy analysis (N = 476) |
|
| Interventions | Each participant treated 1 migraine with oral rizatriptan (5 mg) or placebo within 30 minutes of onset. Up to 2 recurrences could be treated with the same medication. Participants could only treat migraine attacks on days they were not in school or at camp. | |
| Outcomes |
Other reported outcomes:
|
|
| Headache severity scale | 4‐level (none, mild, moderate, severe) | |
| Funding source | Merck | |
| Publication | Journal | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: described as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The number of patients who discontinued prior to taking study medication and the distribution of reasons for the lack of treatment were comparable across the treatment arms." |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
Winner 1997.
| Study characteristics | ||
| Methods | Randomized, double‐blind, placebo‐controlled, 4‐period, outpatient, cross‐over, 4‐attack study of oral sumatriptan | |
| Participants | Participants were 12‐17 yrs with > 6 month history of migraine with or without aura as defined by IHS 1988; had 1‐8 moderate or severe migraine attacks monthly during the 2 months prior to screening Randomized (N = 355); withdrawn (N = 194); intention‐to‐treat and primary efficacy analysis (N = 298) |
|
| Interventions | Each participant treated up to 4 migraine attacks of moderate or severe intensity in a cross‐over fashion; 1 attack with placebo and 3 with sumatriptan 25 mg, 50 mg, or 100 mg (same dose for all 3 attacks). Participants were randomized in a balanced fashion to 1‐12 treatment sequences. | |
| Outcomes |
Other reported outcomes:
|
|
| Headache severity scale | 4‐level (none, mild, moderate, severe) | |
| Funding source | GlaxoSmithKline | |
| Publication | Journal and clinical trial registry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: described as "randomized in a balanced manner to 1 of 12 treatment sequences" |
| Allocation concealment (selection bias) | Unclear risk | Comment: methods to maintain allocation concealment were not described; previous studies by sponsor had adequate allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: described as "double‐blind, placebo‐controlled"; methods to maintain blinding were not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: a total of 194 subjects withdrew, but were balanced across intervention groups |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
Winner 2000.
| Study characteristics | ||
| Methods | Randomized, double‐blind, placebo‐controlled, single‐attack, parallel‐group study of intranasal sumatriptan | |
| Participants | Males and females 12‐17 years of age with a diagnosis of migraine (with or without aura) meeting IHS 1988 criteria and typical duration > 4 h and with at least a 6‐month history of 2‐8 moderate or severe migraine attacks per month for each of the 2 months preceding study enrolment. Participants were required to have < 15 days of tension headache per month and were required to have failed at least 1 previous over‐the‐counter or prescription medication for the treatment of migraine. Randomized (N = 653); withdrawn (N = 147); missing efficacy data (N = 3); intention‐to‐treat analysis (N = 510); primary efficacy analysis (N = 507) |
|
| Interventions | Each participant treated 1 migraine with nasal sumatriptan (5, 10, or 20 mg) or placebo. A second dose of the same nasal spray could be used 2‐24 h after the initial dose. | |
| Outcomes |
Other reported outcomes:
|
|
| Headache severity scale | 4‐level (none, mild, moderate, severe) | |
| Funding source | GlaxoSmithKline | |
| Publication | Journal and clinical trial registry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized after successful screening using a computer‐generated, parallel‐group design, 1:1:1:1 ratio (block size of 8)" |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation schedule was concealed in tamper‐evident, blinded envelopes kept by the sponsor" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "identically appearing placebo" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: missing outcome data balanced across intervention groups |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
Winner 2002.
| Study characteristics | ||
| Methods | Randomized, double‐blind, placebo‐controlled, parallel‐group trial of oral rizatriptan | |
| Participants | Participants were 12‐17 yrs, with an average of at least 1 but no more than 8 migraines per IHS 1988 criteria and stratified in 2 groups: 12‐14 years and 15‐17 years. Efforts were made to enrol equal numbers in each age group. Randomized (N = 360); did not receive study medication (N = 64); primary efficacy analysis (N = 296) |
|
| Interventions | Each participant was instructed to take the study medication (rizatriptan 5 mg or placebo) within 30 min of onset of a moderate or severe migraine | |
| Outcomes |
Other reported outcomes:
|
|
| Headache severity scale | 4‐level (none, mild, moderate, severe) | |
| Funding source | Merck | |
| Publication | Journal | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: small imbalance of missing outcome data in the placebo group (N = 6 vs N = 1) |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported |
Winner 2006.
| Study characteristics | ||
| Methods | Randomized, double‐blind, placebo‐controlled, parallel‐group, multicenter, single‐attack, outpatient study of intranasal sumatriptan | |
| Participants | Participants were 12‐17 years of age; had a history of migraine of at least 6 months duration (with or without aura) in accordance with IHS 1988; had at least 1‐8 moderate or severe migraine attacks per month in each of the 2 months before study enrolment; and were able to distinguish migraine attacks as discrete attacks, separate from other headaches (i.e., tension headaches) Randomized (N = 888); did not receive study medication (N = 150); withdrawn (N = 7); intention‐to‐treat and primary efficacy analysis (N = 731) |
|
| Interventions | Sumatriptan 5 mg nasal spray; sumatriptan 20 mg nasal spray; or placebo | |
| Outcomes |
Other reported outcomes:
|
|
| Headache severity scale | 4‐level (none, mild, moderate, severe) | |
| Funding source | GlaxoSmithKline | |
| Publication | Journal and clinical trial registry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized in a 1:1:1 ratio (in blocks of six) to one of three treatment groups"; "computer‐generated randomization schedule" Comment: previous migraine studies by GlaxoSmithKline have documented acceptable sequence generation (e.g. Winner 2000) |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization schedule prepared by the sponsor" Comment: centralized allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "[nasal spray] devices for active drug and placebo were identical in appearance and construction to maintain the study blind." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all withdrawal documented in a flow diagram |
| Selective reporting (reporting bias) | Low risk | Comment: missing outcome data balanced across intervention groups |
Winner 2007.
| Study characteristics | ||
| Methods | Randomized, double‐blind, parallel‐group, placebo‐controlled trial of oral eletriptan | |
| Participants | Participants were 12‐17 years, met ICHD‐2 criteria for migraine with or without aura, and suffered at least 1 migraine attack every 6 weeks. Mean migraine duration was required to be a minimum of 4 h. Randomized (N = 348); did not receive study medication (N = 74); intention‐to‐treat and primary efficacy analysis (N = 274) |
|
| Interventions | Eletriptan 40 mg PO, placebo taken within 4 h of headache onset | |
| Outcomes |
Other reported outcomes:
|
|
| Headache severity scale | 4‐level (none, mild, moderate, severe) | |
| Funding source | Pfizer | |
| Publication | Journal | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: described as "placebo‐controlled", but no other description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Three hundred and eighty‐four were screened, of whom 348 were randomized to study drug . . ."; "Seventy‐four were randomized but did not take the study drug: (1) 35 did not treat a migraine attack during the 12‐week time window. . ." Comment: withdrawals balanced across intervention groups |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes reported; originally published as an abstract in 2000 (Pitman 2000) followed by this full report. |
AE: adverse events; DHE: dihydroergotamine; LOCF: last observation carried forward; PO: per os (by mouth); VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brousseau 2004 | The Emergency Department setting and parenteral interventions studied (intravenous prochlorperazine versus ketorolac) were significantly different from included clinical trials of interventions in the outpatient setting. As there were few other clinical trials in the Emergency Department setting, we made a post hoc decision to exclude this study to simplify the review conclusions. |
| Cady 2011 | Non‐drug clinical trial and participants were not exclusively pediatric |
| Gertsch 2011 | Open‐label, uncontrolled study |
| NCT00355394 | The Emergency Department setting and parenteral intervention studied (intravenous metoclopramide) was significantly different from included clinical trials of interventions in the outpatient setting. As there were few other clinical trials in the Emergency Department setting, we made a post hoc decision to exclude this study to simplify the review conclusions. |
| Soriani 2001 | A non‐placebo‐controlled randomized study comparing paracetamol and nimesulide. Each of 60 participants in the study treated 10 attacks, and the primary outcome of headache relief was reported as a percentage of attacks. No individual participant data was reported so was not included in the meta‐analysis. |
| SUM40090 | Open‐label, uncontrolled study |
| Trautmann 2010 | Non‐drug clinical trial |
| Winner 2011 | Study of rapid disintegrating formulation of sumatriptan was not a randomized controlled trial |
Characteristics of studies awaiting classification [ordered by study ID]
Winner 2015.
| Methods | Randomized, placebo‐controlled, double‐blind cross‐over trial of oral sumatriptan + naproxen sodium |
| Participants | Male and female adolescents 12‐17 years of age meeting ICHD‐2 criteria with an average of at least 1 but no more than 8 migraines per month in the previous 6 months Enrolled (N = 104); did not receive study medication (N = 10); primary efficacy analysis (N = 94) |
| Interventions | Sumatriptan + naproxen sodium 85 mg + 500 mg; or placebo for the treatment of 4 migraine attacks (3 active, 1 placebo) |
| Outcomes |
Other reported outcomes:
|
| Notes | — |
Differences between protocol and review
At the recommendation of the Cochrane Pain, Palliative and Supportive Care Group, we narrowed the review criteria to include only placebo‐controlled trials. We did not include studies with active comparators or standard of care as the control.
The original protocol outlined a plan to address possible carry‐over and period effects in studies of cross‐over design. Given that migraine is an episodic disorder and that interventions were used to treat discrete migraine episodes, we considered the probability of there being carry‐over and period effects to be very low. Thus, we deemed it inappropriate to exclude studies of cross‐over design as suggested in the Cochrane Handbook for Systematic Reviews of Interventions. We included all studies of cross‐over design even if they did not report the time between treatment periods or a statistical test for carry‐over effect. We performed a sensitivity analysis on study design to assess the influence of cross‐over studies on measures of effect and harm.
We changed the allowed ages from the range of 3 to 18 years to any age under 18. For the subgroup analysis, we defined age groups according to custom in these types of studies (i.e. children less than 12 years of age; adolescents 12 to 17 years), as these age groups represent distinct ages whereby treatment may differ. No minimum age was included, as the diagnosis of migraine using appropriate criteria will preclude diagnosis in the very young, in whom criteria are not met.
All studies reported the treatment of a single attack. The original protocol outlined a plan to address studies that reported the treatment of multiple headache episodes, but it was not necessary to implement this. If required, we will apply this plan in future revisions of the study.
We added 'pain‐free' as the primary outcome measure, given that it is a recommended primary outcome measure (Tfelt‐Hansen 2012), it is the most desirable outcome for patients, and most studies report it.
We changed 'headache relief' from a primary to a secondary outcome measure.
No studies reported ordinal outcomes, continuous outcomes, or group mean change scores. Thus, we did not implement these aspects of the protocol, but we will if required in future revisions of the review.
We removed photophobia and phonophobia from secondary outcome measures as recommended by reviewers and often only inferred from behaviour.
We removed patient preference for treatment as a secondary outcome measure.
We did not include assessment time points beyond two hours post medication administration given the known relatively short duration of migraine in many children and adolescents.
We included a primary outcome measure to assess harm (i.e. any adverse events), as per the updated version 5.1.0 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We revised the search strategy to improve search terms and focus on pharmacological interventions.
We did not include a high versus low methodological quality score based on the Jadad scale in the sensitivity analysis, as we used the 'Risk of bias' tables to assess methodological quality. We used allocation concealment as per the protocol, as it is one of the domains in the 'Risk of bias' assessment.
We added the GRADE assessment of quality of evidence.
The original protocol proposed calculating within‐patient improvement scores whenever the required data were reported, but these were not available.
We added reports in a peer‐reviewed indexed journal to the sensitivity analysis.
We did not identify or include any quasi‐randomized studies in the review. Future revisions of the review would not benefit from their inclusion, so we removed this selection criterion as suggested by reviewers.
We amended the title from "Drugs for treating acute migraine headaches in children and adolescents" to "Drugs for the acute treatment of migraine in children and adolescents" for clarity.
Contributions of authors
Lawrence Richer ‐ protocol development, review of publications, data entry, analysis, report writing, presentation of findings, updates to report.
Meghan Linsdell ‐ review of publications, data abstraction, data entry, review of report, updates to report.
Lori Billinghurst ‐ protocol development, review and selection of publications, presentation of findings.
Kelly Russell ‐ data abstraction, report writing, review of report.
Ben Vandermeer ‐ data abstraction, statistical analysis, report writing.
Tamara Durec and Ellen Crumley ‐ development of publication database search.
Lisa Hartling ‐ protocol development, review of report.
Terry Klassen ‐ protocol development, review of report.
Sources of support
Internal sources
Department of Pediatrics, University of Alberta, Canada
Alberta Research Centre for Child Health Evidence, Canada
External sources
Stollery Children's Hospital Foundation, Canada
American Academy of Pediatrics Resident Research Grant, USA
Declarations of interest
Lawrence Richer: no relevant conflicts of interest to declare.
Lori Billinghurst: no relevant conflicts of interest to declare.
Kelly Russell: no relevant conflicts of interest to declare.
Ben Vandermeer: no relevant conflicts of interest to declare.
Tamara Durec: no relevant conflicts of interest to declare.
Ellen Crumley: no relevant conflicts of interest to declare.
Lisa Hartling: no relevant conflicts of interest to declare.
Terry Klassen: no relevant conflicts of interest to declare.
Meghan Linsdell: no relevant conflicts of interest to declare.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Ahonen 2004 {published data only}
- Ahonen K, Hämäläinen ML, Rantala H, Hoppu K. Nasal sumatriptan is effective in treatment of migraine attacks in children: a randomized trial. Neurology 2004;62(6):883-7. [DOI: 10.1212/01.WNL.0000115105.05966.A7] [DOI] [PubMed] [Google Scholar]
Ahonen 2006 {published data only}
- Ahonen K, Hämäläinen ML, Eerola M, Hoppu K. A randomized trial of rizatriptan in migraine attacks in children. Neurology 2006;67(7):1135-40. [DOI: 10.1212/01.wnl.0000238179.79888.44] [DOI] [PubMed] [Google Scholar]
Callenbach 2007 {published and unpublished data}SUM30042
- Callenbach PM, Pels LP, Mulder PG, Linssen WH, Gooskens RH, Van der Zwan JL, et al. Sumatriptan nasal spray in the acute treatment of migraine in adolescents and children. European Journal of Paediatric Neurology 2007;11(6):325-30. [DOI: 10.1016/j.ejpn.2007.02.010] [DOI] [PubMed] [Google Scholar]
- SUM30042. A multi-centre, randomised, double-blind, placebo-controlled, cross-over study to investigate the efficacy, safety and tolerability of sumatriptan nasal spray (10mg or 20mg) in the treatment of migraine in patients aged 12-17. http://www.gsk-clinicalstudyregister.com/study/SUM30042#rs (accessed 24 June 2015).
Derosier 2012 {published and unpublished data}
- Derosier FJ, Lewis D, Hershey AD, Winner PK, Pearlman E, Rothner AD, et al. Randomized trial of sumatriptan and naproxen sodium combination in adolescent migraine. Pediatrics 2012;129(6):e1411-20. [DOI: 10.1542/peds.2011-2455] [DOI] [PubMed] [Google Scholar]
Evers 2006 {published data only}
- Evers S, Rahmann A, Kraemer C, Kurlemann G, Debus O, Husstedt IW, et al. Treatment of childhood migraine attacks with oral zolmitriptan and ibuprofen. Neurology 2006;67(3):497-9. [DOI: 10.1212/01.wnl.0000231138.18629.d5] [DOI] [PubMed] [Google Scholar]
Fujita 2014 {published and unpublished data}SUM111035
- Fujita M, Sato K, Nishioka H, Sakai F. Oral sumatriptan for migraine in children and adolescents: a randomized, multicenter, placebo-controlled, parallel group study. Cephalalgia 2014;34(5):365-75. [DOI: 10.1177/0333102413510213] [DOI] [PubMed] [Google Scholar]
- SUM111035. A randomized, multicenter, placebo-controlled, parallel group study to evaluate the efficacy and safety of oral sumatriptan for the acute treatment of migraine in children and adolescents. http://www.gsk-clinicalstudyregister.com/study/111035#ps (accessed 24 June 2015).
Hämäläinen 1997a {published data only}
- Hämäläinen ML, Hoppu K, Valkeila E, Santavuori P. Ibuprofen or acetaminophen for the acute treatment of migraine in children: a double-blind, randomized, placebo-controlled, crossover study. Neurology 1997;48(1):103-7. [DOI: 10.1212/WNL.48.1.103] [DOI] [PubMed] [Google Scholar]
Hämäläinen 1997b {published data only}
- Hämäläinen ML, Hoppu K, Santavuori P. Sumatriptan for migraine attacks in children: a randomized placebo-controlled study. Do children with migraine respond to oral sumatriptan differently from adults? Neurology 1997;48(4):1100-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hämäläinen 1997c {published data only}
- Hämäläinen M, Hoppu K, Santavuori PR. Oral dihydroergotamine for therapy-resistant migraine attacks in children. Pediatric Neurology 1997;16(2):114-7. [DOI: 10.1016/S0887-8994(96)00289-5] [DOI] [PubMed] [Google Scholar]
Hämäläinen 2002 {published and unpublished data}SUM30009
- Hämäläinen M, Jones M, Loftus J, Saiers J. Sumatriptan nasal spray for migraine: a review of studies in patients aged 17 years and younger. International Journal of Clinical Practice 2002;56(9):704-9. [PMID: ] [PubMed] [Google Scholar]
- SUM30009. A single centre, placebo-controlled, double blind, randomised cross-over, single attack study evaluating the efficacy and tolerability of sumatriptan nasal spray 10 mg for the acute treatment of migraine in children suffering from refractory migraine with/without aura. http://www.gsk-clinicalstudyregister.com/study/SUM30009#rs (accessed 24 June 2015).
Ho 2012 {published data only}
- Ho TW, Pearlman E, Lewis D, Hämäläinen M, Connor K, Michelson D, et al. Efficacy and tolerability of rizatriptan in pediatric migraineurs: results from a randomized, double-blind, placebo-controlled trial using a novel adaptive enrichment design. Headache 2012;32(10):750-65. [DOI: 10.1177/0333102412451358] [DOI] [PubMed] [Google Scholar]
Lewis 2002 {published data only}
- Lewis DW, Kellstein D, Dahl G, Burke B, Frank LM, Toor S, et al. Children's ibuprofen suspension for the acute treatment of pediatric migraine. Headache 2002;42(8):780-6. [DOI: 10.1046/j.1526-4610.2002.02180.x] [DOI] [PubMed] [Google Scholar]
Lewis 2007 {published data only}
- Lewis DW, Winner P, Hershey AD, Waslewski WW, Adolescent Migraine Steering Committee. Efficacy of zolmitriptan nasal spray in adolescent migraine. Pediatrics 2007;120(2):390-6. [DOI: 10.1542/peds.2007-0085] [DOI] [PubMed] [Google Scholar]
Linder 2008 {published and unpublished data}
- Linder SL, Mathew NT, Cady RK, Finlayson G, Ishkanian G, Lewis DW. Efficacy and tolerability of almotriptan in adolescents: a randomized, double-blind, placebo-controlled trial. Headache 2008;48(9):1326-36. [DOI: 10.1111/j.1526-4610.2008.01138.x] [DOI] [PubMed] [Google Scholar]
NCT01211145 {published and unpublished data}NCT01211145
- NCT01211145. Zomig - treatment of acute migraine headache in adolescents (TEENZ). clinicaltrials.gov/ct2/show/NCT01211145 (accessed 24 June 2015).
Rothner 1997 {published and unpublished data}S2WA3012
- Rothner A, Edwards K, Kerr L, DeBussey S, Asgharnejad M. Efficacy and safety of naratriptan tablets in adolescent migraine. Journal of Neurological Sciences 1997;150(Suppl 1):S106. [Google Scholar]
- S2WA3012. A randomized, double-blind, placebo-controlled, parallel study to evaluate the efficacy, safety and tolerability of oral naratriptan in an adolescent migraine population. http://www.gsk-clinicalstudyregister.com/study/S2WA3012#rs (accessed 24 June 2015).
Rothner 1999a {published and unpublished data}SUMB2003
- Rothner D, Asgharnejad M. Tolerability of sumatriptan tablets in the acute treatment of migraine in adolescent patients: a review of data from clinical trials. European Journal of Neurology 1999;6(Suppl 3):106. [Google Scholar]
- SUMB2003. A double-blind, randomised, placebo-controlled study to compare the efficacy and safety of oral sumatriptan (25mg, 50mg and 100mg) in the acute treatment of migraine in adolescents. http://www.gsk-clinicalstudyregister.com/study/SUMB2003#rs (accessed 24 June 2015).
Rothner 1999b {published and unpublished data}S2CT37
- Rothner D, Asgharnejad M. Tolerability of sumatriptan tablets in the acute treatment of migraine in adolescent patients: a review of data from clinical trials. European Journal of Neurology 1999;6(Suppl 3):106. [Google Scholar]
Rothner 1999c {published and unpublished data}S2CT40
- Rother D, Asgharnejad M. Tolerability of sumatriptan tablets in the acute treatment of migraine in adolescent patients: a review of data from clinical trials. European Journal of Neurology 1999;6(Suppl 3):106. [GSK ID: S2CT40] [Google Scholar]
Rothner 2006 {published data only}
- Rothner AD, Wasiewski W, Winner P, Lewis D, Stankowski J. Zolmitriptan oral tablet in migraine treatment: high placebo responses in adolescents. Headache 2006;46(1):101-9. [DOI: 10.1111/j.1526-4610.2006.00313.x] [DOI] [PubMed] [Google Scholar]
Ueberall 1999 {published data only}
- Ueberall MA, Wenzel D. Intranasal sumatriptan for the acute treatment of migraine in children. Neurology 1999;52(7):1507-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Visser 2004a {published data only}
- Visser WH, Winner P, Strohmaier K, Klipfel M, Peng Y, McCarroll K, et al. Rizatriptan 5 mg for the acute treatment of migraine in adolescents: results from a double-blind, single-attack study and two open-label, multiple-attack studies. Headache 2004;44:891-9. [DOI: 10.1111/j.1526-4610.2004.04171.x] [DOI] [PubMed] [Google Scholar]
Winner 1997 {published and unpublished data}SUMA2002
- Winner P, Prensky A, Linder S, DeBussey S, Asgharnejad M. Adolescent migraine: efficacy and safety of sumatriptan tablets. Journal of the Neurological Sciences 1997;150(Suppl 1):S172. [DOI: 10.1016/S0022-510X(97)85694-8] [DOI] [Google Scholar]
Winner 2000 {published and unpublished data}SUMA3005
- Winner P, Rothner AD, Saper J, Nett R, Asgharnejad M, Laurenza A, et al. A randomized, double-blind, placebo-controlled study of sumatriptan nasal spray in the treatment of acute migraine in adolescents. Pediatrics 2000;106(5):989-97. [PMID: ] [DOI] [PubMed] [Google Scholar]
Winner 2002 {published data only}
- Winner P, Lewis D, Visser WH, Jiang K, Ahrens S, Evans JK, et al. Rizatriptan 5 mg for the acute treatment of migraine in adolescents: a randomized, double-blind, placebo-controlled study. Headache 2002;42(1):49-55. [DOI: 10.1046/j.1526-4610.2002.02013.x] [DOI] [PubMed] [Google Scholar]
Winner 2006 {published data only}
- SUM30045. A double-blind, placebo-controlled, parallel group study to evaluate two dose levels (5mg And 20mg) of sumatriptan nasal spray in the acute treatment of a single migraine attack in adolescent migraineurs (12-17 years of age). http://www.gsk-clinicalstudyregister.com/study/SUM30045#rs (accessed 24 June 2015).
- Winner P, Rothner AD, Wooten JD, Webster C, Ames M. Sumatriptan nasal spray in adolescent migraineurs: a randomized, double-blind, placebo-controlled, acute study. Headache 2006;46(2):212-22. [DOI: 10.1111/j.1526-4610.2006.00339.x] [DOI] [PubMed] [Google Scholar]
- Winner P, Rothner D, Webster CJ, Ames MH, Kori SH. Randomized, double-blind, placebo- controlled study of sumatriptan nasal spray in adolescent migraineurs. Neurology 2004;62(7 Suppl 5):A182. [Google Scholar]
Winner 2007 {published data only}
- Pitman V. Efficacy, safety and tolerability of oral eletriptan (40 mg) for the treatment of migraine in adolescents (12-17 years). Headache 2000;40(5):424-5. [Google Scholar]
- Winner P, Liner SL, Lipton RB, Almas M, Parsons B, Pitman V. Eletriptan for the acute treatment of migraine in adolescents: results of a double-blind, placebo-controlled trial. Headache 2007;47(4):511-8. [DOI: 10.1111/j.1526-4610.2007.00755.x] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Brousseau 2004 {published data only}
- Brousseau DC, Duffy SJ, Anderson AC, Linakis JG. Treatment of pediatric migraines: a randomized, double-blind trial of prochlorperazine versus ketorolac. Annals of Emergency Medicine 2004;43(2):256-62. [DOI: 10.1016/S0196-0644(03)00716-9] [DOI] [PubMed] [Google Scholar]
Cady 2011 {published data only}
- Cady RK, Goldstein J, Nett R, Mitchell R, Beach ME, Browning R. A double-blind placebo-controlled pilot study of sublingual feverfew and ginger (LipiGesicTM M) in the treatment of migraine. Headache 2011;51(7):1078-86. [DOI: 10.1111/j.1526-4610.2011.01910.x] [DOI] [PubMed] [Google Scholar]
Gertsch 2011 {published data only}
- Gertsch EA, Loharuka S, Wolter-Warmerdam KG, Tong S, Kedia S. Intravenous magnesium as abortive treatment for headaches in children. Annals of Neurology 2011;70(S15):S167. [DOI: 10.1002/ana.22563] [DOI] [Google Scholar]
NCT00355394 {unpublished data only}
- NCT00355394. Treatment of acute migraine headache in children. clinicaltrials.gov/show/NCT00355394 (accessed 3 February 2016).
Soriani 2001 {published data only}
- Soriani S, Battistella PA, Naccarella C, Tozzi E, Fiumana E, Fanaro S. Nimesulide and acetaminophen for the treatment of juvenile migraine: a study for comparison of efficacy, safety, and tolerability. Headache Quarterly 2001;12:233-6. [Google Scholar]
SUM40090 {published data only}
- SUM40090. The use of oral sumatriptan on compassionate grounds for the acute treatment of migraine in adolescent patients (ages 12 to 17). http://www.gsk-clinicalstudyregister.com/study/SUM40090#rs (accessed 24 June 2015).
Trautmann 2010 {published data only}
- Trautmann E, Kroner-Herwig B. A randomized controlled trial of Internet-based self-help training for recurrent headache in childhood and adolescence. Behaviour Research & Therapy 2010;48(1):28-37. [DOI: 10.1016/j.brat.2009.09.004] [DOI] [PubMed] [Google Scholar]
Winner 2011 {published data only}
- Winner P. Sumatriptan formulated with RT technologyTM as early intervention for migraine in adolescents. Journal of Pediatric Neurology 2011;9(2):169-75. [DOI: 10.3233/JPN-2011-0471] [DOI] [Google Scholar]
References to studies awaiting assessment
Winner 2015 {published and unpublished data}
- NCT01016678. Treximet Early Intervention Adolescent Migraine (TEAM). clinicaltrials.gov/ct2/show/NCT01016678 (accessed 24 June 2015).
- Winner P, Linder S, Hershey AD. Consistency of response to sumatriptan/naproxen sodium in a randomized placebo-controlled, cross-over study for the acute treatment of migraine in adolescents. Headache 2015;55:519-28. [DOI: 10.1111/head.12555] [DOI] [PubMed] [Google Scholar]
Additional references
Bille 1997
- Bille B. A 40-year follow-up of school children with migraine. Cephalalgia 1997;17(4):488-91; discussion 487. [DOI: 10.1046/j.1468-2982.1997.1704488.x] [DOI] [PubMed] [Google Scholar]
Bonfert 2013
- Bonfert M, Straube A, Schroeder AS, Reilich P, Ebinger F, Heinen F. Primary headache in children and adolescents: update on pharmacotherapy of migraine and tension-type headache. Neuropediatrics 2013;44(1):3-19. [DOI: 10.1055/s-0032-1330856] [DOI] [PubMed] [Google Scholar]
Curtin 2002
- Curtin F, Elbourne D, Altman DG. Meta-analysis combining parallel and cross-over clinical trials. II: binary outcomes. Statistics in Medicine 2002;21(15):2145-59. [DOI: 10.1002/sim.1206] [DOI] [PubMed] [Google Scholar]
Damen 2005
- Damen L, Bruijn JK, Verhagen AP, Berger MY, Passchier J, Koes BW. Symptomatic treatment of migraine in children: a systematic review of medication trials. Pediatrics 2005;116(2):e295-302. [DOI: 10.1542/peds.2004-2742] [DOI] [PubMed] [Google Scholar]
Davies 2000
- Davies GM, Santanello N, Lipton R. Determinants of patient satisfaction with migraine therapy. Cephalalgia 2000;20(6):554-60. [DOI: 10.1046/j.1468-2982.2000.00082.x] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI: 10.1136/bmj.315.7109.629] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eiland 2010
- Eiland LS, Hunt MO. The use of triptans for pediatric migraines. Paediatric Drugs 2010;12(6):379-89. [DOI: 10.2165/11532860-000000000-00000] [DOI] [PubMed] [Google Scholar]
Goadsby 2008
- Goadsby PJ. The 'Act when Mild' (AwM) study: a step forward in our understanding of early treatment in acute migraine. Cephalalgia 2008;28(Suppl 2):36-41. [DOI: 10.1111/j.1468-2982.2008.01689.x] [DOI] [PubMed] [Google Scholar]
GRADEPro 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.) GRADEpro Guideline Development Tool. McMaster University (developed by Evidence Prime, Inc.), 2015. Available from www.gradepro.org.
Harbord 2006
- Harbord RM, Egger M, Sterne JAC. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443-57. [DOI: 10.1002/sim.2380] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. [DOI: 10.1002/sim.1186] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from: www.cochrane-handbook.org.
ICHD‐2
- Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders, 2nd edition. Cephalalgia 2004;24(1 Suppl):1-160. [DOI: 10.1111/j.1468-2982.2003.00823.x] [DOI] [PubMed] [Google Scholar]
ICHD‐3 beta
- Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33(9):629-808. [DOI: 10.1177/0333102413485658] [DOI] [PubMed] [Google Scholar]
IHS 1988
- International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias, and facial pain. Cephalalgia 1988;8(Suppl 7):1-96. [PubMed] [Google Scholar]
Lathyris 2007
- Lathyris DN, Trikalinos TA, Ioannidis JP. Evidence from crossover trials: empirical evaluation and comparison against parallel arm trials. International Journal of Epidemiology 2007;36(2):422-30. [DOI: 10.1093/ije/dym001] [DOI] [PubMed] [Google Scholar]
Levy 2008
- Levy D, Zhang X-C, Jakubowski M, Burstein R. Sensitization of meningeal nociceptors: inhibition by naproxen. European Journal of Neuroscience 2008;27(4):917-22. [DOI: 10.1111/j.1460-9568.2008.06068.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 2004
- Lewis D, Ashwal S, Hershey A, Hirtz D, Yonker M, Silberstein S. Practice parameter: pharmacological treatment of migraine headache in children and adolescents: report of the American Academy of Neurology Quality Standards Subcommittee and the Practice Committee of the Child Neurology Society. Neurology 2004;63(12):2215-24. [DOI: 10.1212/01.WNL.0000147332.41993.90] [DOI] [PubMed] [Google Scholar]
Lipton 2002
- Lipton RB, Hamelsky SW, Dayno JM. What do patients with migraine want from acute migraine treatment? Headache 2002;42(Suppl 1):3-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Major 2003
- Major PW, Grubisa HSI, Thie NMR. Triptans for treatment of acute pediatric migraine: a systematic literature review. Pediatric Neurology 2003;29(5):425-9. [DOI: 10.1016/S0887-8994(03)00400-4] [DOI] [PubMed] [Google Scholar]
Pierce 2010
- Pierce CA, Voss B. Efficacy and safety of ibuprofen and acetaminophen in children and adults: a meta-analysis and qualitative review. Annals of Pharmacotherapy 2010;44(3):489-506. [DOI: 10.1345/aph.1M332] [DOI] [PubMed] [Google Scholar]
Powers 2003
- Powers SW, Patton SR, Hommel KA, Hershey AD. Quality of life in childhood migraines: clinical impact and comparison to other chronic illnesses. Pediatrics 2003;112(1 Pt 1):e1-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Powers 2004
- Powers SW, Patton SR, Hommel KA, Hershey AD. Quality of life in paediatric migraine: characterization of age-related effects using PedsQL 4.0. Cephalalgia 2004;24(2):120-7. [DOI: 10.1111/j.1468-2982.2004.00652.x] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Review Manager. Version 5.3. The Nordic Cochrane Centre, Copenhagen: Cochrane, 2014.
Silver 2008
- Silver S, Gano D, Gerretsen P. Acute treatment of paediatric migraine: a meta-analysis of efficacy. Journal of Paediatrics & Child Health 2008;44(1-2):3-9. [DOI: 10.1111/j.1440-1754.2007.01206.x] [DOI] [PubMed] [Google Scholar]
Stata 14 [Computer program]
- Stata Statistical Software: Release 14. StataCorp. College Station, TX: StataCorp LP, 2015.
Stovner 2007
- Stovner LJ, Hagen K, Jensen R, Katsarava Z, Lipton RB, Scher AI, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia 2007;27(3):193-210. [DOI: 10.1111/j.1468-2982.2007.01288.x] [DOI] [PubMed] [Google Scholar]
Sun 2013
Suthisisang 2010
- Suthisisang CC, Poolsup N, Suksomboon N, Lertpipopmetha V, Tepwitukgid B. Meta-analysis of the efficacy and safety of naproxen sodium in the acute treatment of migraine. Headache 2010;50(5):808-18. [DOI: 10.1111/j.1526-4610.2010.01635.x] [DOI] [PubMed] [Google Scholar]
Tfelt‐Hansen 2012
- Tfelt-Hansen P, Pascual J, Ramadan N, Dahlof C, D'Amico D, Diener H-C, et al. Guidelines for controlled trials of drugs in migraine: third edition. A guide for investigators. Cephalalgia 2012;32(1):6-38. [DOI: 10.1177/0333102411417901] [DOI] [PubMed] [Google Scholar]
Toldo 2012
- Toldo I, De Carlo D, Bolzonella B, Sartori S, Battistella PA. The pharmacological treatment of migraine in children and adolescents: an overview. Expert Review of Neurotherapeutics 2012;12(9):1133-42. [DOI: 10.1586/ern.12.104] [DOI] [PubMed] [Google Scholar]
Vollono 2011
- Vollono C, Vigevano F, Tarantino S, Valeriani M. Triptans other than sumatriptan in child and adolescent migraine: literature review. Expert Review of Neurotherapeutics 2011;11(3):395-401. [DOI: 10.1586/ern.10.147] [DOI] [PubMed] [Google Scholar]
Winner 1995
- Winner P, Martinez W, Mate L, Bello L. Classification of pediatric migraine: proposed revisions to the IHS criteria. Headache 1995;35(7):407-10. [DOI] [PubMed] [Google Scholar]
Wöber‐Bingöl 2013
- Wöber-Bingöl Ç. Pharmacological treatment of acute migraine in adolescents and children. Paediatric Drugs 2013;15(3):235-46. [DOI: 10.1007/s40272-013-0019-3] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Richer 2009
- Richer LP. Practice Variation in the Treatment of Children with Migraine in the Emergency Department [MSc Thesis]. Edmonton, AB: University of Alberta, 2009. [Google Scholar]


