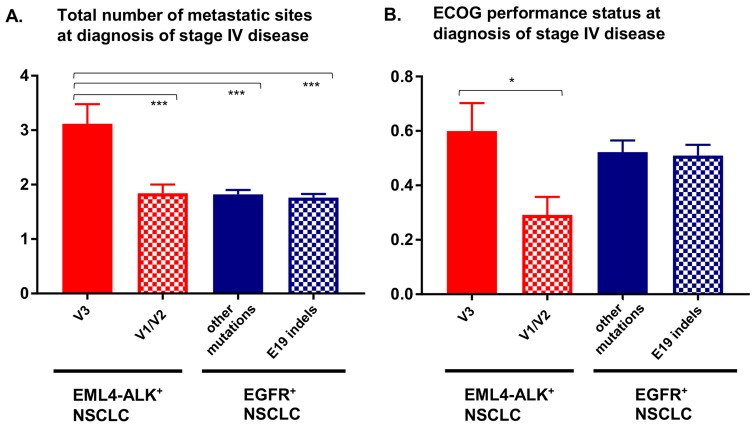
Figure 1. Number of metastatic sites and performance status in newly diagnosed stage IV EML4-ALK+ and EGFR+ NSCLC patients.
A. The total number of metastatic sites (intrathoracic, brain, liver, bone, adrenal, other) for newly diagnosed stage IV EML4-ALK+ (n = 34 V3 cases, n = 44 V1/V2 cases) and stage IV EGFR+ (n = 221 EGFR exon 19 [E19] indel cases, n = 197 cases with other EGFR mutations) NSCLC patients typed at our institution with available data [18,22]. Statistical comparison was performed with ANOVA (p < 0.001) followed by the Dunnett’s post-hoc test. Columns and error bars show mean values and their standard errors: 3.12 and 0.36 for EML4-ALK V3, 1.84 and 0.16 for EML4-ALK V1/V2, 1.76 and 0.07 for EGFR exon 19 indels, 0.82 and 0.08 for other EGFR alterations. Statistically significant results are shown in the graph; ***: p < 0.001. B. The Eastern Cooperative Oncology Group (ECOG) performance status for newly diagnosed stage IV EML4-ALK+ (n = 35 V3 cases, n = 48 V1/V2 cases) and EGFR+ (n = 210 EGFR exon 19 [E19] indel cases, n = 178 with other EGFR mutations) NSCLC patients from our institution with available data [18,22]. Statistical comparison was performed with ANOVA (p < 0.05) followed by the Dunnett’s post-hoc test. Columns and error bars show mean values and their standard errors: 0.60 and 0.10 for EML4-ALK V3, 0.29 and 0.07 for EML4-ALK V1/V2, 0.52 and 0.04 for EGFR exon 19 indels, 0.51 and 0.04 for other EGFR alterations); *:p = 0.037.