Abstract
Background
Naloxone, a specific opioid antagonist, is available for the treatment of newborn infants with cardiorespiratory or neurological depression that may be due to intrauterine exposure to opioid. It is unclear whether newborn infants may benefit from this therapy and whether naloxone has any harmful effects.
Objectives
To determine the effect of naloxone on the need for and duration of neonatal unit stay in infants of mothers who received opioid analgesia prior to delivery or of mothers who have used a prescribed or non‐prescribed opioid during pregnancy.
Search methods
We searched the following databases in February 2018: the Cochrane Central Register of Controlled Trials (the Cochrane Library 2018, Issue 1), MEDLINE (OvidSP), MEDLINE In process & Other Non‐Indexed Citations (OvidSP), Embase (OvidSP), CINAHL (EBSCO), Maternity and Infant Care (OvidSP), and PubMed. We searched for ongoing and completed trials in the WHO International Clinical Trials Registry Platform and the EU Clinical Trials Register. We checked the reference lists of relevant articles to identify further potentially relevant studies.
Selection criteria
Randomised controlled trials comparing the administration of naloxone versus placebo, or no drug, or another dose of naloxone to newborn infants with suspected or confirmed in utero exposure to opioid.
Data collection and analysis
We extracted data using the standard methods of Cochrane Neonatal with separate evaluation of trial quality and data extraction by two review authors and synthesis of data using risk ratio, risk difference, and mean difference.
Main results
We included nine trials, with 316 participants in total, that compared the effects of naloxone versus placebo or no drug in newborn infants exposed to maternal opioid analgesia prior to delivery. None of the included trials investigated infants born to mothers who had used a prescribed or non‐prescribed opioid during pregnancy. None of these trials specifically recruited infants with cardiorespiratory or neurological depression. The main outcomes reported were measures of respiratory function in the first six hours after birth. There is some evidence that naloxone increases alveolar ventilation. The trials did not assess the effect on the primary outcomes of this review (admission to a neonatal unit and failure to establish breastfeeding).
Authors' conclusions
The existing evidence from randomised controlled trials is insufficient to determine whether naloxone confers any important benefits to newborn infants with cardiorespiratory or neurological depression that may be due to intrauterine exposure to opioid. Given concerns about the safety of naloxone in this context, it may be appropriate to limit its use to randomised controlled trials that aim to resolve these uncertainties.
Plain language summary
Naloxone for opioid‐exposed newborn infants
Review question
Does naloxone (a drug that counters the negative effects of opioids on breathing) help newborn babies whose mothers have received opioid pain relief during birth?
Background
When a woman receives opioid medicines for pain relief during labour (for example, pethidine, morphine, and similar drugs), the opioid can cross over to the baby inside the womb and then reduce the newborn baby's breathing rate. Naloxone, a drug that counters the effects of opioids, can be given to the newborn baby to try to prevent or treat problems with breathing. This may reduce the chance of the baby needing to go to a neonatal unit for help with breathing, and reduce the need for separating mother and baby (and so help with establishing breastfeeding). Concern exists, however, that naloxone may cause side effects, including possible long‐term developmental problems.
Study characteristics
We found nine completed trials that compared giving to newborn babies, whose mothers had received opioids during labour, either naloxone or a placebo ('dummy drug'). These trials were conducted more than 30 years ago and they were generally very small including only about 300 infants in total. Most of the trials did not use reliable methods consistently. Evidence is up‐to‐date as of February 2018.
Key results
The trials reported the effects of naloxone on the baby's breathing but did not assess the effect on the need for babies to be cared for in a neonatal unit (separated from their mother), whether they needed help with breathing, or on breastfeeding success. None of the trials assessed long‐term development. We did not find any trials including babies born to mothers who had used opioids (whether prescribed or non‐prescribed) during pregnancy.
Quality of evidence
The available evidence was not sufficient to determine whether giving naloxone to babies whose mothers received opioids during birth was helpful or harmful.
Summary of findings
Summary of findings for the main comparison. Naloxone compared to placebo or no drug for opiate‐exposed newborn infants.
| Naloxone compared to placebo or no drug for opioid‐exposed newborn infants | ||||||
| Patient or population: opioid‐exposed newborn infants Setting: Intervention: naloxone Comparison: placebo or no drug | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo or no drug | Risk with Naloxone | |||||
| Admission to neonatal intensive or special care unit ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Primary outcome, not measured in the included trials |
| Duration of neonatal intensive or special care unit stay ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Primary outcome, not measured in the included trials |
| Failure to establish breastfeeding prior to hospital discharge ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Primary outcome, not measured in the included trials |
| Neurodevelopmental outcomes beyond infancy ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Primary outcome, not measured in the included trials |
| Receipt of endotracheal intubation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Secondary outcome, not measured in the included trials |
| Receipt of assisted ventilation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Secondary outcome, not measured in the included trials |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
Background
Description of the condition
Opioid analgesics are available, recommended, and commonly used for relief of maternal pain during labour in most birth settings in middle‐ and high‐income countries (Jones 2012; NICE 2017). Estimates suggest that about one‐third to one‐half of women receive opioid analgesia in labour. The extent of use in lower‐middle and low‐income settings is less certain (Redshaw 2007; Ullman 2010), though likely to be less than in high‐income countries (James 2012; Ogboli‐Nwasor 2014).
The commonly used opioids (pethidine, diamorphine, morphine, fentanyl) are highly lipid soluble and transplacental transfer to the fetus occurs rapidly. Intrauterine exposure to maternal opioids within about four hours of birth may be associated with neurological and cardiorespiratory depression and feeding‐behaviour problems in newborn infants (Kumar 2003; Mercer 2007; Reynolds 2010) Concern exists that these adverse effects may delay neonatal physiological transition and postnatal adaptation and result in neonatal unit admission and potentially separation of the infant and mother. Delay in establishment of effective breastfeeding is an important possible consequence of this maternal‐infant separation (Burchell 2016; Nissen 1995; Ransjo‐Arvidson 2001). Observational data from surveillance studies have suggested that infants who experience an acute life‐threatening event in the early neonatal period or a sudden unexpected early neonatal death are more likely to have been exposed to maternal opiates during birth, but a causal link has not been established (Lutz 2016).
Description of the intervention
Naloxone, a specific "opioid antagonist" that blocks the actions of opiates on cells, is available for the treatment of neurological and cardiorespiratory depression in opioid‐exposed newborn infants. The International Liaison Committee on Resuscitation (ILCOR) has provided guidance on the use of naloxone for newborn infants (Niermeyer 2001). The advice follows the long‐standing recommendation of the American Academy of Pediatrics (AAP) Committee on Drugs that naloxone should not be used routinely in opioid‐exposed newborn infants but should be "reserved for adjunctive therapy in selected infants who have not initiated or established independent respiration following ventilation, are significantly depressed, and have a high probability of being narcotized" (AAP 1980). These recommendations refer to infants of mothers who have received opioid for analgesia up to four hours prior to delivery. The dose of naloxone recommended in 1980, 0.01 mg/kg, was later revised to 0.1 mg/kg (AAP 1990). In 2010, an ILCOR Consensus on Science and Treatment Recommendations statement updated this advice and suggested that naloxone should only be given to infants with severe respiratory depression after positive‐pressure ventilation has restored a normal heart rate and colour (Kattwinkel 2010; Perlman 2010). Despite these guidelines, surveys of policy and practice indicate that clinicians continue to administer naloxone to opioid‐exposed newborn infants in the first few minutes after birth even in the absence of neurological or cardiorespiratory depression or before effective supported ventilation is established (Herschel 2000; Gill 2007).
Opioid‐dependent mothers
The AAP Committee on Drugs has advised that naloxone should not be administered to infants of opioid‐dependent mothers as naloxone may precipitate acute withdrawal and seizures in opioid‐habituated infants (Gibbs 1989; AAP 1998). There are few data on significant adverse events due to naloxone in infants of opioid‐dependent mothers and some authors have recommended a small dose of naloxone (0.01 mg/kg) as a part of the resuscitation of such infants (Maas 1990).
Why it is important to do this review
Naloxone should not be regarded as harmless. Concern exists that naloxone may interfere with the role of the infant's own natural opioids in programming metabolic, homonal, and physiological processes (Smotherman 1992; De Castro 1993; Szeto 1995). Given these questions of appropriateness of use and potential long‐term effects, it is important to evaluate the available data on the use of naloxone in opioid‐exposed newborn infants.
Objectives
To determine the effect of naloxone on the need for and duration of neonatal unit stay in infants of mothers who received opioid analgesia prior to delivery or of mothers who have used a prescribed or non‐prescribed opioid during pregnancy.
Methods
Criteria for considering studies for this review
Types of studies
Controlled trials using either random or quasi‐random patient allocation.
Types of participants
Newborn infants of any gestation with suspected or confirmed exposure to opioids, either:
as maternal pain relief prior to delivery;
as a result of use during pregnancy.
Types of interventions
Trials comparing naloxone with placebo or no drug, or comparing more than one dose of naloxone, as part of the management of newborn infants.
Types of outcome measures
Primary outcomes
Mother‐infant separation, effect on breastfeeding, and neurodevelopment
Admission to neonatal intensive or special care unit;
Duration of neonatal intensive or special care unit stay;
Failure to establish breastfeeding by hospital discharge;
Neurodevelopmental outcomes beyond infancy assessed using validated assessment tools.
Secondary outcomes
Cardio‐respiratory function, need for support, and neurobehavioural outcomes
Measures of respiratory function, such as Apgar score, or arterial blood pH, or arterial or alveolar carbon dioxide tension measured within the first six hours after birth;
Receipt of assisted ventilation (any form of mechanical ventilation including continuous positive airway pressure) in the neonatal period;
Receipt of endotracheal intubation for respiratory support;
Duration of assisted ventilation (days);
Duration of endotracheal intubation (days);
Days from birth to establish full oral feeds independently of parenteral fluids or nutrition or of enteral tube feeding;
Features of opioid withdrawal, using validated behavioural assessment measures in the neonatal period;
Seizures in the neonatal period.
Search methods for identification of studies
We used the standard search strategy of Cochrane Neonatal.
Electronic searches
We updated the searches in February 2018 to identify reports of trials available since the searches in June 2012. The original search strategy from June 2012 was checked and updated (Appendix 1). We searched the following databases on 20 February 2018: the Cochrane Central Register of Controlled Trials (the Cochrane Library 2018, Issue 1), MEDLINE (OvidSP), MEDLINE In process & Other Non‐Indexed Citations (OvidSP), Embase (OvidSP), CINAHL (EBSCO), Maternity and Infant Care (OvidSP), and PubMed. We imported the search results into reference management software and de‐duplicated the results against the previous search results from June 2012. We did not apply any language restrictions. A search filter was applied in MEDLINE and Embase to limit retrieval to randomised controlled trials (Lefebvre 2008; Lefebvre 2011).
We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization’s International Trials Registry and Platform www.whoint/ictrp/search/en/, the ISRCTN Registry, and the EU Clinical Trials Register https://www.clinicaltrialsregister.eu/). We were unable to search the metaRegister of Controlled Trials for this update as it is no longer available.
Searching other resources
We examined the references in studies identified as potentially relevant for other eligible studies. We also searched the abstracts from the annual meetings of the Pediatric Academic Societies (1993 to 2018), the European Society for Pediatric Research (1995 to 2017), the UK Royal College of Paediatrics and Child Health (2000 to 2018) and the Perinatal Society of Australia and New Zealand (2000 to 2017). We considered trials reported only as abstracts to be eligible if sufficient information was available from the report, or from contact with the authors, to fulfil the inclusion criteria.
Data collection and analysis
We used the standard methods of Cochrane Neonatal.
Selection of studies
Two review authors independently screened the title and abstract of all studies identified by the above search strategy. We assessed the full text of any potentially eligible reports and excluded those studies that did not meet all of the inclusion criteria. We discussed any disagreements until consensus was achieved.
Data extraction and management
We used a data collection form to extract relevant information from each included study. Two review authors extracted the data separately. We discussed any disagreements with the third review author until we reached consensus.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool (Higgins 2017). Any disagreements were resolved by discussion or by a third assessor. See Appendix 2 for a description of risk of bias for each domain assessed.
Measures of treatment effect
We calculated risk ratio (RR) and risk difference (RD) for dichotomous data and mean difference (MD) for continuous data, with respective 95% confidence intervals (CI) using RevMan 2014 software. When it was deemed appropriate to combine two or more study arms, we obtained the treatment effects from the combined data using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Unit of analysis issues
The unit of analysis was the participating infant in individually randomised trials and the neonatal unit (or subunit) for cluster‐randomised RCTs. For cluster‐randomised RCTs, we planned to undertake analyses at the level of the individual while accounting for the clustering in the data using the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
We requested outcome data from the trial investigators when these were unavailable in the report.
Assessment of heterogeneity
Two authors assessed clinical heterogeneity, with a meta‐analysis conducted only when both authors agreed that study participants, interventions, and outcomes were sufficiently similar.
We examined the treatment effects of individual trials and heterogeneity between trial results by inspecting the forest plots. We calculated the I² statistic for each analysis to quantify inconsistency across studies and described the percentage of variability in effect estimates that may be due to heterogeneity rather than to sampling error. If we detected moderate or high heterogeneity (I² > 50%), we would explore the possible causes (for example, differences in study design, participants, interventions, or completeness of outcome assessments).
Assessment of reporting biases
We intended to conduct a funnel‐plot analysis if there were data from more than 10 trials included in a meta‐analysis.
Data synthesis
We used fixed‐effect models for meta‐analysis (as per Cochrane Neonatal recommendations). Where moderate or high heterogeneity existed, we planned to examine the potential causes in subgroup and sensitivity analyses.
Quality of evidence
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to assess the quality of evidence for the primary outcomes and key secondary outcomes we considered most relevant to parents and caregivers (Schünemann 2013; Appendix 3):
Admission to neonatal intensive or special care unit;
Duration of neonatal intensive or special care unit stay;
Failure to establish breastfeeding by hospital discharge;
Neurodevelopmental outcomes beyond infancy;
Receipt of assisted ventilation;
Receipt of endotracheal intubation.
Two authors independently assessed the quality of the evidence for each of these outcomes. We considered evidence from randomised controlled trials initially as high‐quality but downgraded this evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of the evidence.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses:
dose of naloxone < 0.1 mg/kg body weight;
dose of naloxone ≥ 0.1 mg/kg body weight.
Sensitivity analysis
We planned sensitivity analyses to determine if the findings were affected by including only studies of adequate methodology (low risk of bias), defined as adequate randomisation and allocation concealment, blinding of intervention and measurement, and < 10% loss to follow‐up.
Results
Description of studies
Results of the search
The update search returned 588 records. After de‐duplication, 120 titles and abstracts were screened. The records that did not meet the inclusion criteria were excluded at title and abstract screening, and no studies were ordered for full‐text screening. The study selection process is illustrated in Figure 1. We did not identify any planned or ongoing studies.
1.
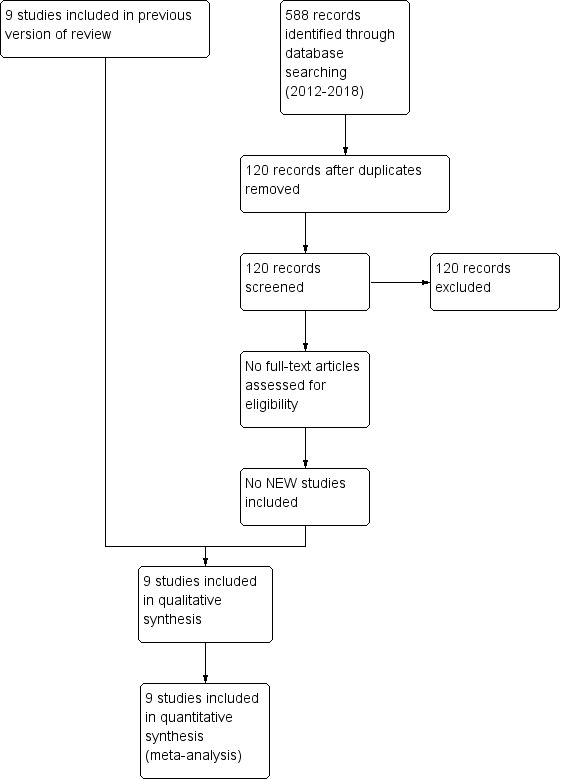
Study flow diagram: review update
Included studies
We included nine trials and these are described in the table 'Characteristics of included studies'. Two of these were reported in the same article (Dick 1978a; Dick 1978b).
A total of 316 infants participated in the included trials. All were undertaken in the 1970s or early 1980s. The participants were term newborn infants whose mothers had received pethidine (meperidine) for pain relief up to six hours prior to delivery. None of the trials specifically restricted participation to infants with cardiorespiratory or neurological depression following opioid exposure. In most trials, the intervention appears to have been given in the first five minutes after birth. In two trials, naloxone was given later; at 30 minutes (Gerhardt 1977) or at one hour (Welles 1984) after birth. Naloxone was administered via the intramuscular route in four trials, and in the other five via the umbilical venous route. The dose of naloxone used ranged from 0.01 to 0.07 mg/kg with the exception of one study in which a total dose of 0.2 mg was given (Wiener 1977b). The outcomes most commonly assessed were measures of respiratory effort such as Apgar scores, blood gases values, or other measures of alveolar ventilation.
None of the trials examined the effects of naloxone in infants of mothers who had used a prescribed or non‐prescribed narcotic during pregnancy.
Excluded studies
We excluded two studies (Martin 1972; Brice 1979b; see Characteristics of excluded studies).
Risk of bias in included studies
All of the trials were small and none presented a sample size calculation. The 'Risk of bias' assessment was hampered by incomplete reporting of trial methods (most likely due to the studies pre‐dating current reporting guidelines). Across included studies, several 'Risk of bias' domains had to be assessed as 'unclear' as details were not reported in the publications.
Allocation
Three studies reported adequate measures of allocation concealment and random sequence generation, reducing the risk of selection bias in these trials. The remaining six reports did not provide sufficient details of measures to ensure allocation concealment or random sequence generation.
Blinding
Caregivers or assessors were likely to have been blinded in five of the trials. Three trials reported that outcome assessors were unblinded and in one trial it was unclear whether outcome assessors were blinded.
Incomplete outcome data
All of the trials appear to have achieved complete or near‐complete follow‐up of infants recruited although none of the trials undertook follow‐up beyond the first three days after birth.
Selective reporting
The risk of reporting bias was unclear across the included studies as trial protocols were not available (most likely due to the age of the studies), However, for eight of the nine included trials, there was no reason to suspect selective reporting.
One trial was considered to be at high risk of selective reporting as the paper stated that certain outcomes (including one primary and one secondary outcome) would not be reported in the publication but would be available from the corresponding author. Due to the age of the study, we were unable to make contact with any of the authors and were, hence, unable to access these data despite knowing that they were collected during the trial.
Other potential sources of bias
In seven out of nine included studies, no other potential sources of bias were identified and the risk of other bias was, consequently, judged to be low. One study was stopped early because the randomisation code was broken. However, further details pertaining to the causes of this breach of protocol or its consequences were not discussed in the paper. One other study was reported to have been funded by the pharmaceutical company that manufactured the intervention. Both these trials were judged to have an unclear risk of other biases.
Effects of interventions
See: Table 1
Primary outcomes
Admission to neonatal intensive or special care unit:
Not reported.
Duration of neonatal intensive or special care unit stay:
Not reported.
Failure to establish breastfeeding by hospital discharge:
Not reported.
Neurodevelopmental outcomes beyond infancy assessed using validated assessment:
Not reported.
Secondary outcomes
Measures of respiratory function:
Eight of the trials presented data on measures of respiratory function measured within the first six hours after birth. There were no statistically significant differences in the Apgar score (Bonta 1979), respiratory rate (Evans 1976), time to sustained respiration (Brice 1979a), minute ventilation (Gerhardt 1977), or blood gas parameters (Bonta 1979; Dick 1978a; Dick 1978b).
Four trials assessed measures of alveolar ventilation (Brice 1979a; Evans 1976; Wiener 1977a; Wiener 1977b). At 30 minutes and four hours post intervention, the expired carbon dioxide output and alveolar ventilation rate were statistically significantly higher, and the alveolar carbon dioxide tension lower, in the naloxone group (Analysis 1.1; Analysis 1.2; Analysis 1.3; Figure 2; Figure 3; Figure 4). We detected statistical heterogeneity in analysis 1.2 (alveolar carbon dioxide tension) and analysis 1.3 (alveolar ventilation). In each analysis (alveolar carbon dioxide tension at 30 minutes (1.2.2) and at four hours (1.2.4) and alveolar ventilation at 30 minutes (1.3.3) and at four hours (1.3.5)), the heterogeneity arose from the same study (Wiener 1977b). This study used a larger dose of naloxone, and naloxone was given intramuscularly compared with intravenously in the other studies. In the analyses of alveolar carbon dioxide tension at 30 minutes and four hours (1.2.2 and 1.2.4), Wiener 1977b showed a greater effect size in favour of naloxone compared to the other studies included in these analyses. The same was true for analyses of alveolar ventilation at 30 minutes and four hours.
1.1. Analysis.
Comparison 1 Naloxone versus placebo or no drug, Outcome 1 Expired carbon dioxide output (mL/kg/min).
1.2. Analysis.
Comparison 1 Naloxone versus placebo or no drug, Outcome 2 Alveolar carbon dioxide tension (kPa).
1.3. Analysis.
Comparison 1 Naloxone versus placebo or no drug, Outcome 3 Alveolar ventilation (mL/kg/minute).
2.
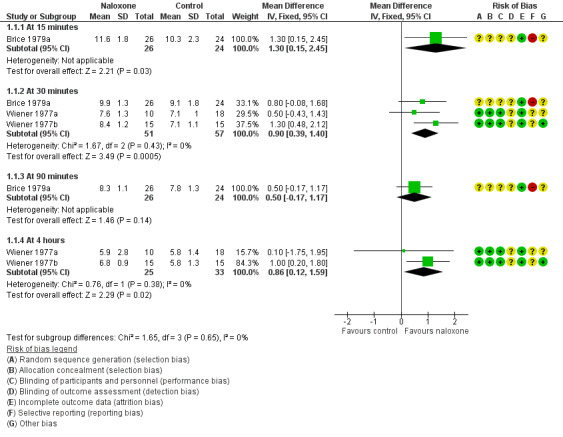
Forest plot of comparison: 1 Naloxone versus placebo or no drug, outcome: 1.1 Expired carbon dioxide output (mL/kg/min).
3.
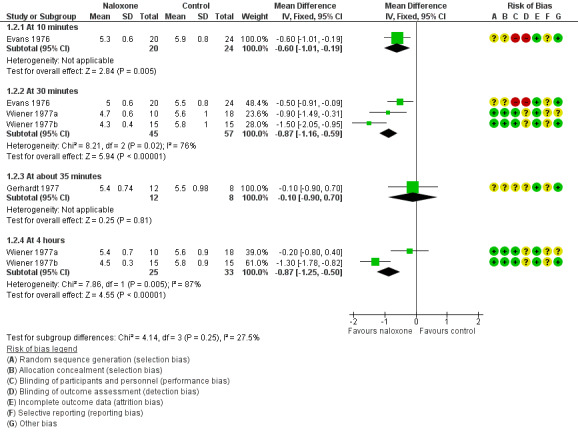
Forest plot of comparison: 1 Naloxone versus placebo or no drug, outcome: 1.2 Alveolar carbon dioxide tension (kPa).
4.
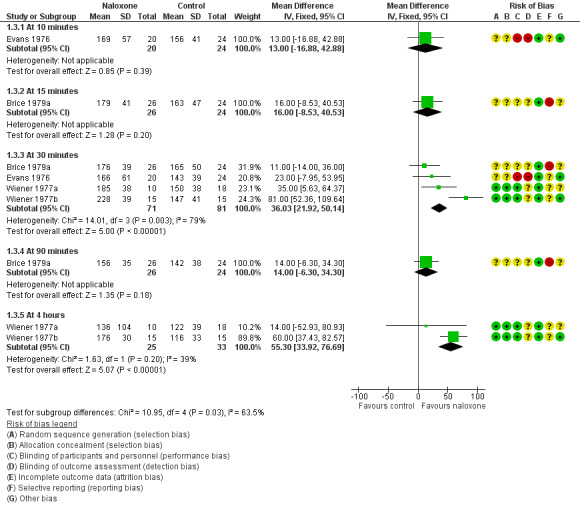
Forest plot of comparison: 1 Naloxone versus placebo or no drug, outcome: 1.3 Alveolar ventilation (mL/kg/minute).
Receipt of assisted ventilation in the neonatal period:
Not reported.
Receipt of endotracheal intubation for respiratory support:
Not reported.
Duration of assisted ventilation:
Not reported.
Duration of endotracheal intubation: not reported.
Days from birth to establish full oral feeds independently of parenteral fluids or nutrition or of enteral tube feeding:
Not reported.
Features of opioid withdrawal:
The studies that reported the Scanlon Behavioural Score (Bonta 1979; Brice 1979a) and the Brazelton Neonatal Behavioural Assessment Score (Brice 1979a; Welles 1984) did not find any statistically significant differences. One trial reported the Broussard Neonatal Perception Inventory at 72 hours and found statistically significantly "less optimal behaviour" in the naloxone group (Welles 1984). Standard deviations were not reported in any of these studies. Wiener 1977b found that the time taken to habituate to a sound‐specific stimulus within the first 48 hours was statistically significantly lower in infants who received intramuscular naloxone versus placebo. Wiener 1977a stated that there were no "important differences" in habituation to auditory stimulus between infants who received intravenous naloxone versus placebo.
Seizures in the neonatal period:
Not reported.
Subgroup analyses
Dose of naloxone < 0.1 mg/kg body weight:
All of the included trials used this dose.
Dose of naloxone ≥ 0.1 mg/kg body weight:
None of the included trials used this dose.
Sensitivity analyses
Only one trial report was assessed as having an overall 'low risk of bias' defined as adequate randomisation and allocation concealment, blinding of intervention and measurement, and < 10% loss to follow‐up (Bonta 1979). Data from this trial were not included in any of the meta‐analyses.
Discussion
Summary of main results
We identified nine small trials that compared naloxone versus placebo or no drug for treating newborn infants who had been exposed to maternal opioid analgesia prior to delivery. Our update search in 2018 identified no trials investigating naloxone in this context. The trials evaluated the effect of naloxone on infants exposed to opioid analgesia in labour. None examined the use of naloxone in infants who had been exposed to opioid in utero during pregnancy, for example, due to maternal opioid‐dependence.
None of the included trials provided any data on any of the primary outcomes of this review, that is, maternal separation (need for neonatal unit admission), effect on breastfeeding, and neurodevelopment beyond infancy. With regard to secondary outcome measures, most trials only reported measures of respiratory function and neurological behaviour in the first 48 hours after birth. The analyses of measures of alveolar carbon dioxide tension and alveolar ventilation were affected by statistical heterogeneity caused by one study showing greater effect sizes in favour of naloxone (Wiener 1977b). This could be attributed to the greater dose of naloxone used in this study and possibly also to the fact that naloxone was given via the intramuscular route in this study (compared to intravenous delivery in all other studies). When given intravenously, naloxone is absorbed more effectively but has a shorter half‐life compared with intramuscular administration (Perlman 2010).
Overall completeness and applicability of evidence
The trials provided some evidence that infants who received naloxone had higher indices of alveolar ventilation, higher expired carbon dioxide levels, and lower alveolar carbon dioxide tensions than control infants. The clinical significance of these findings is unclear since the infants recruited to the trials did not appear to have been selected because of cardiorespiratory depression. Infants with low Apgar scores for up to five minutes were not eligible for inclusion in two trials (Bonta 1979; Welles 1984). There were no trial data that assessed whether treatment with naloxone affected the need for, or the duration of, mechanical respiratory support including positive‐pressure ventilation. All of the included trials were conducted over 30 years ago. The evidence is likely to be of limited relevance in the clinical context for which naloxone is recommended by the ILCOR, that is, for opioid‐exposed newborn infants with respiratory depression despite appropriate ventilation (Kattwinkel 2010; Niermeyer 2001; Perlman 2010). The ILCOR Consensus on Science rates neurodevelopmental measures as a critical outcome, reflecting the high importance clinicians, policy makers, and parents place on this outcome. None of the trials included in this review reported any neurodevelopmental measures beyond infancy.
Quality of the evidence
All of the trials were small and had various methodological weaknesses including uncertain allocation concealment that may have biased their findings and the findings of this review (Figure 5). Ascertainment or surveillance biases may also be present since caregivers and clinicians in some of the trials were aware of the allocated intervention. Although follow‐up assessment was complete in the trials, none assessed outcomes beyond the first few days after birth.
5.
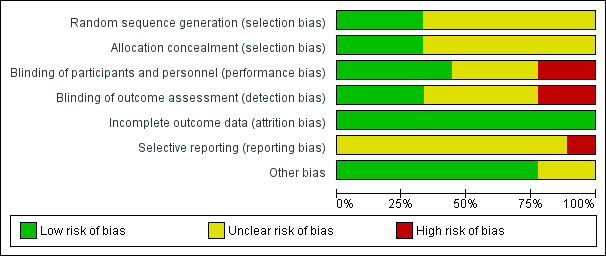
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Potential biases in the review process
Following standard Cochrane methods, we tried to minimise the risk of biases introduced during the review process. The literature search was conducted by an information specialist with the aim to identify and retrieve all relevant evidence published since the last update of this review in 2012. We searched conference abstracts and reference lists of potentially eligible publications. There remains a risk that studies could have been missed due to indexing errors in the databases. Throughout the review process, we followed methods to minimise the risk of reviewer error and bias (study selection carried out independently and in duplicate).
Agreements and disagreements with other studies or reviews
The 2010 ILCOR Consensus on Science (Kattwinkel 2010; Perlman 2010) does not recommend naloxone as part of the initial resuscitation of newborn infants. This recommendation is based largely on the same body of evidence as this review. The current ILCOR Consensus on Science published in 2015 (Perlman 2015) does not mention naloxone at all, reflecting the lack of new evidence in this area.
Authors' conclusions
Implications for practice.
The available trial data do not provide any evidence that administration of naloxone to infants exposed in utero to opioid during delivery affects any important outcomes. The efficacy and safety of naloxone for infants chronically exposed in utero to opioids in opioid‐dependent women has not been assessed.
Implications for research.
Clinicians and service‐users may consider it appropriate to undertake a randomised controlled trial to determine if naloxone confers any important benefits to newborn infants with cardiorespiratory or neurological depression that may be due to intrauterine exposure to opioids. This trial should assess outcomes that are relevant to the infant, family, and caregivers, such as the need for admission to a neonatal unit for ongoing respiratory support. In view of the concerns that naloxone may interfere with the role of endogenous opioids in neuroendocrine programming and on behaviour (De Castro 1993; Smotherman 1992; Szeto 1995), follow‐up assessment beyond infancy should determine neurodevelopmental outcomes which have been judged to be of critical importance by ILCOR (Perlman 2015).
There are no trials concerning the use of naloxone for the treatment of infants that were chronically exposed to opioids in utero, most likely due to concerns that administration of naloxone might cause seizures in these infants (AAP 1998; Gibbs 1989).
What's new
| Date | Event | Description |
|---|---|---|
| 9 May 2018 | New search has been performed | This updates the review "Naloxone for opioid‐exposed newborn infants" (Moe‐Byrne 2013). |
| 9 May 2018 | New citation required but conclusions have not changed | Search updated in February 2018. No new trials added. Background and discussion updated. Risk of Bias tables updated. Summary of findings table added. |
History
Protocol first published: Issue 1, 2002 Review first published: Issue 4, 2002
| Date | Event | Description |
|---|---|---|
| 31 July 2012 | New citation required but conclusions have not changed | Search updated in June 2012.
No new trials added. Background and discussion updated. |
| 10 June 2008 | Amended | Converted to new review format. |
| 14 March 2007 | New search has been performed | This review updates "Naloxone for narcotic‐exposed newborn infants", published in the Cochrane Database of Systematic Reviews, The Cochrane Library, Issue 4, 2002 (McGuire 2002). Our electronic search was updated in February 2007. No new trials that fulfilled eligibility criteria were identified. We re‐categorised studies that were previously listed as "studies awaiting assessment". Four of these were abstracts presenting data that was also presented in included substantive publications. These are now listed as secondary publications. One study was excluded. |
| 18 June 2002 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Melissa Harden, Information Specialist, provided invaluable assistance in devising and running the electronic search.
Appendices
Appendix 1. Electronic search strategy
Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL)
Wiley http://onlinelibrary.wiley.com/
Issue 1 of 12, January 2018
Searched on: 20th February 2018
Records retrieved: 14
#1 MeSH descriptor: [Infant, Newborn] explode all trees 15665
#2 MeSH descriptor: [Premature Birth] this term only 597
#3 (neonat* or neo next nat*):ti,ab 14017
#4 (newborn* or new next born* or newly next born*):ti,ab 6237
#5 (preterm or preterms or pre next term or pre next terms):ti,ab 9039
#6 (preemie* or premie or premies):ti,ab 21
#7 (prematur* near/3 (birth* or born or deliver*)):ti,ab 1118
#8 (low near/3 (birthweight* or birth next weight*)):ti,ab 3505
#9 (lbw or vlbw or elbw):ti,ab 1221
#10 infan*:ti,ab 27709
#11 (baby or babies):ti,ab 4644
#12 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 44720
#13 MeSH descriptor: [Naloxone] explode all trees 1833
#14 naloxone:ti,ab 1826
#15 narcan:ti,ab 5
#16 MeSH descriptor: [Narcotic Antagonists] this term only 1129
#17 ((narcotic or opiate or opioid) near/3 antagonist*):ti,ab 1031
#18 #13 or #14 or #15 or #16 or #17 3280
#19 #12 and #18 76
#20 #12 and #18 in Trials 68
#21 #12 and #18 Publication Year from 2007 to 2018 14
Line #20 shows the number of hits in CENTRAL only.
Key
MeSH descriptor = indexing term (MeSH heading)
* = truncation
:ti,ab = terms in either title or abstract fields
NEAR/3 = terms within three words of each other (any order)
NEXT = terms are next to each other
CINAHL
via EBSCO http://www.ebsco.com/
Inception to 19th February 2018
Searched on: 20th February 2018
Records retrieved: 83
S1 MH "Infant, Newborn+" 107,105
S2 MH "Infant, Low Birth Weight+" 10,646
S3 MH "Infant, Premature" 17,476
S4 (MH "Childbirth, Premature") 7,235
S5 TI ( neonat* or neo‐nat* ) OR AB ( neonat* or neo‐nat* ) 45,560
S6 TI ( newborn* or new‐born* or newly N1 born* ) OR AB ( newborn* or new‐born* or newly N1 born* ) 21,724
S7 TI ( preterm or preterms or pre‐term or pre‐terms ) OR AB ( preterm or preterms or pre‐term or pre‐terms) 22,267
S8 TI ( preemie* or premie or premies ) OR AB ( preemie* or premie or premies ) 239
S9 TI ( prematur* N3 (birth* or born or deliver*) ) OR AB ( prematur* N3 (birth* or born or deliver*) ) 3,179
S10 TI ( low N3 (birthweight* or birth‐weight*) ) OR AB ( low N3 (birthweight* or birth‐weight*) ) 8,753
S11 TI ( lbw or vlbw or elbw ) OR AB ( lbw or vlbw or elbw ) 2,359
S12 TI infan* OR AB infan* 79,927
S13 TI ( baby or babies ) OR AB ( baby or babies ) 23,527
S14 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 190,257
S15 (MH "Naloxone+") 3,344
S16 TI naloxone OR AB naloxone 1,874
S17 TI narcan OR AB narcan 38
S18 (MH "Narcotic Antagonists") 1,598
S19 TI ( ((narcotic or opiate or opioid) N3 antagonist*) ) OR AB ( ((narcotic or opiate or opioid) N3 antagonist*) ) 796
S20 S15 OR S16 OR S17 OR S18 OR S19 4,922
S21 S14 AND S20 140
S22 S14 AND S20 Limiters ‐ Publication Year: 2007‐2018 83
Key
MH = indexing term (CINAHL heading)
+ = exploded CINAHL heading
* = truncation
TI = words in the title
AB = words in the abstract
N3 = terms within three words of each other (any order)
Embase
OvidSP http://ovidsp.ovid.com/
1974 to 2018 February 18
Searched on: 19th February 2018
Records retrieved: 50
A search strategy developed by Lefebvre et. al. to identify randomised trials in Embase was used to limit retrieval to clinical trials (lines 23‐37) (Lefebvre 2008).
1 exp infant/ (983099)
2 newborn/ (535610)
3 prematurity/ (91829)
4 premature labor/ (40137)
5 exp low birth weight/ (53021)
6 (neonat$ or neo nat$).ti,ab. (302625)
7 (newborn$ or new born$ or newly born$).ti,ab. (184612)
8 (preterm or preterms or pre term or pre terms).ti,ab. (86396)
9 (preemie$ or premie or premies).ti,ab. (214)
10 (prematur$ adj3 (birth$ or born or deliver$)).ti,ab. (18958)
11 (low adj3 (birthweight$ or birth weight$)).ti,ab. (38393)
12 (lbw or vlbw or elbw).ti,ab. (9710)
13 infan$.ti,ab. (460096)
14 (baby or babies).ti,ab. (85499)
15 or/1‐14 (1360576)
16 naloxone/ (39568)
17 naltrexone/ (13293)
18 naloxone.ti,ab. (25894)
19 narcan.ti,ab. (120)
20 narcotic antagonist/ (2171)
21 ((narcotic or opiate or opioid) adj3 antagonist$).ti,ab. (13795)
22 16 or 17 or 18 or 19 or 20 or 21 (58022)
23 random$.ti,ab. (1269278)
24 factorial$.ti,ab. (32048)
25 crossover$.ti,ab. (64838)
26 cross‐over$.ti,ab. (28769)
27 placebo$.ti,ab. (268419)
28 (doubl$ adj blind$).ti,ab. (186242)
29 (singl$ adj blind$).ti,ab. (20605)
30 assign$.ti,ab. (330112)
31 allocat$.ti,ab. (123986)
32 volunteer$.ti,ab. (228781)
33 Crossover Procedure/ (54337)
34 double blind procedure/ (146460)
35 Randomized Controlled Trial/ (487494)
36 single blind procedure/ (30408)
37 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 (1963083)
38 15 and 22 and 37 (141)
39 animal/ (1835634)
40 exp animal experiment/ (2167842)
41 nonhuman/ (5322101)
42 (rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. (5689708)
43 39 or 40 or 41 or 42 (8403753)
44 exp human/ (19255041)
45 human experiment/ (396693)
46 44 or 45 (19256570)
47 43 not (43 and 46) (6322301)
48 38 not 47 (119)
49 limit 48 to yr="2007 ‐Current" (50)
Key:
/ = indexing term (EMTREE heading)
exp = exploded EMTREE heading
$ = truncation
.ti,ab. = terms in either title or abstract fields
adj3 = terms within three words of each other (any order)
.sh. = terms in the EMTREE heading field
Maternity and Infant Care
OvidSP http://ovidsp.ovid.com/
1971 to December 2017
Searched on: 20th February 2018
Records retrieved: 65
1 (neonat$ or neo nat$).mp. (42157)
2 (newborn$ or new born$ or newly born$).mp. (38013)
3 (preterm or preterms or pre term or pre terms).mp. (23364)
4 (preemie$ or premie or premies).mp. (50)
5 (prematur$ adj3 (birth$ or born or deliver$)).mp. (6532)
6 (low adj3 (birthweight$ or birth weight$)).mp. (10998)
7 (lbw or vlbw or elbw).mp. (2765)
8 infan$.mp. (82242)
9 (baby or babies).mp. (27270)
10 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 (117850)
11 naloxone.mp. (74)
12 Naltrexone.mp. (10)
13 narcan.mp. (1)
14 ((narcotic or opiate or opioid) adj3 antagonist$).mp. (20)
15 11 or 12 or 13 or 14 (91)
16 10 and 15 (65)
Key
$ = truncation
.mp. = multi‐purpose field search – includes terms in either title, abstract, keyword heading, name of substance, original title or subject heading fields
adj3 = terms within three words of each other (any order)
MEDLINE
OvidSP http://ovidsp.ovid.com/
1946 to February Week 2 2018
Searched on: 19th February 2018
Records retrieved: 89
The Cochrane highly sensitive search strategy for identifying randomized trials in MEDLINE (sensitivity‐maximizing version) was used to limit retrieval to clinical trials (lines 19‐29) (Lefebvre 2011).
1 exp Infant, Newborn/ (560906)
2 Premature Birth/ (10524)
3 (neonat$ or neo nat$).ti,ab. (214766)
4 (newborn$ or new born$ or newly born$).ti,ab. (140975)
5 (preterm or preterms or pre term or pre terms).ti,ab. (55474)
6 (preemie$ or premie or premies).ti,ab. (137)
7 (prematur$ adj3 (birth$ or born or deliver$)).ti,ab. (12830)
8 (low adj3 (birthweight$ or birth weight$)).ti,ab. (28169)
9 (lbw or vlbw or elbw).ti,ab. (6466)
10 infan$.ti,ab. (360691)
11 (baby or babies).ti,ab. (56440)
12 or/1‐11 (903657)
13 exp Naloxone/ (23951)
14 naloxone.ti,ab,rn. (24723)
15 narcan.ti,ab,rn. (57)
16 Narcotic Antagonists/ (12351)
17 ((narcotic or opiate or opioid) adj3 antagonist$).ti,ab. (11217)
18 or/13‐17 (35523)
19 randomized controlled trial.pt. (453101)
20 controlled clinical trial.pt. (92131)
21 randomized.ab. (352150)
22 placebo.ab. (170252)
23 drug therapy.fs. (1991268)
24 randomly.ab. (244938)
25 trial.ab. (364753)
26 groups.ab. (1531866)
27 or/19‐26 (3828607)
28 exp animals/ not humans/ (4424434)
29 27 not 28 (3265481)
30 12 and 18 and 29 (233)
31 limit 30 to yr="2007 ‐Current" (89)
Key
/ = indexing term (MeSH heading)
exp = exploded MeSH heading
$ = truncation
.ti,ab. = terms in either title or abstract fields
adj3 = terms within three words of each other (any order)
.pt.= terms in the publication type field
.fs.= floating subheading
MEDLINE (Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily)
OvidSP http://ovidsp.ovid.com/
16th February 2018
Searched on: 19th February 2018
Records retrieved: 27
1 exp Infant, Newborn/ (436)
2 Premature Birth/ (16)
3 (neonat$ or neo nat$).ti,ab. (19923)
4 (newborn$ or new born$ or newly born$).ti,ab. (10342)
5 (preterm or preterms or pre term or pre terms).ti,ab. (7080)
6 (preemie$ or premie or premies).ti,ab. (8)
7 (prematur$ adj3 (birth$ or born or deliver$)).ti,ab. (1180)
8 (low adj3 (birthweight$ or birth weight$)).ti,ab. (2673)
9 (lbw or vlbw or elbw).ti,ab. (727)
10 infan$.ti,ab. (31472)
11 (baby or babies).ti,ab. (6278)
12 or/1‐11 (56812)
13 exp Naloxone/ (13)
14 naloxone.ti,ab,rn. (993)
15 narcan.ti,ab,rn. (6)
16 Narcotic Antagonists/ (13)
17 ((narcotic or opiate or opioid) adj3 antagonist$).ti,ab. (526)
18 or/13‐17 (1307)
19 12 and 18 (27)
PubMed
http://www.ncbi.nlm.nih.gov/pubmed/
Searched on: 20th February 2018
Records retrieved: 246
The Cochrane highly sensitive search strategy for identifying randomized trials in PubMed (sensitivity‐maximizing version) was used to limit retrieval to clinical trials (Lefebvre 2011).
Search ((((((((((((((((((("Infant, Newborn"[Mesh])) OR ("Premature Birth"[Mesh])) OR (((neonat*[Title/Abstract]) OR neo nat*[Title/Abstract]) OR neo‐nat*[Title/Abstract])) OR (((((newborn*[Title/Abstract]) OR new born*[Title/Abstract]) OR new‐born*[Title/Abstract]) OR newly born*[Title/Abstract]) OR newly‐born*[Title/Abstract])) OR ((((((preterm[Title/Abstract]) OR preterms[Title/Abstract]) OR pre term[Title/Abstract]) OR pre‐term[Title/Abstract]) OR pre terms[Title/Abstract]) OR pre‐terms[Title/Abstract])) OR (((preemie*[Title/Abstract]) OR premie[Title/Abstract]) OR premies[Title/Abstract])) OR ((prematur*[Title/Abstract]) AND birth*[Title/Abstract])) OR ((prematur*[Title/Abstract]) AND born[Title/Abstract])) OR ((prematur*[Title/Abstract]) AND deliver*[Title/Abstract])) OR ((low[Title/Abstract]) AND birthweight*[Title/Abstract])) OR ((low[Title/Abstract]) AND birth weight*[Title/Abstract])) OR ((low[Title/Abstract]) AND birth‐weight*[Title/Abstract])) OR (((lbw[Title/Abstract]) OR vlbw[Title/Abstract]) OR elbw[Title/Abstract])) OR (infan*[Title/Abstract])) OR ((baby[Title/Abstract]) OR babies[Title/Abstract]))) AND (((((((("Naloxone"[Mesh])) OR (naloxone)) OR (narcan)) OR ((narcotic[Title/Abstract]) AND antagonist*[Title/Abstract])) OR ((opiate[Title/Abstract]) AND antagonist*[Title/Abstract])) OR ("Narcotic Antagonists"[Mesh:noexp])) OR ((opioid[Title/Abstract]) AND antagonist*[Title/Abstract])))) AND (((((((((((randomized controlled trial[Publication Type])) OR (controlled clinical trial[Publication Type])) OR (randomized[Title/Abstract])) OR (placebo[Title/Abstract])) OR (drug therapy[MeSH Subheading])) OR (randomly[Title/Abstract])) OR (trial[Title/Abstract])) OR (groups[Title/Abstract]))) NOT (animals[mh] NOT humans[mh]))
Key
[Mesh] = exploded indexing term (MeSH heading)
[mh] = exploded indexing term (MeSH heading)
[Mesh:NoExp] = indexing term (MeSH heading) not exploded
* = truncation
[Title/Abstract]) = terms in either title or abstract fields
[Publication Type] = terms in the publication type field
[MeSH Subheading] = MeSH subheading
Trial registers
ClinicalTrials.gov
http://www.clinicaltrials.gov/
Searched on: 20th February 2018
Records retrieved: 5
1. Naloxone AND (infant OR infants OR newborn OR newborns OR premature OR prematurity OR neonate OR neonates OR neonatal OR preterm OR preterms OR preemie OR preemies OR premie OR premies OR birthweight OR baby OR babies) – 3 studies
2. Narcan AND (infant OR infants OR newborn OR newborns OR premature OR prematurity OR neonate OR neonates OR neonatal OR preterm OR preterms OR preemie OR preemies OR premie OR premies OR birthweight OR baby OR babies) – 2 studies
WHO International Clinical Trials Registry Platform
http://apps.who.int/trialsearch/AdvSearch.aspx
Searched on: 20th February 2018
Records retrieved: 9
1. Naloxone in title, clinical trials in children – 5 results
2. Naloxone in intervention field, clinical trials in children – 4 results
3. Narcan in title, clinical trials in children – 0
4. Narcan in intervention field, clinical trials in children – 0 results
EU Clinical Trials Register
https://www.clinicaltrialsregister.eu/ctr‐search/search
Searched on: 20th February 2018
Records retrieved: 0
1. naloxone textword search, limited to newborn or preterm new born infants age filter – 0 results
2. narcan textword search, limited to newborn or preterm new born infants age filter – 0 results
Appendix 2. Risk of bias tool
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table; computer random number generator);
high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk, high risk or unclear risk for participants; and
low risk, high risk or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion, where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
6. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it was clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review had been reported);
high risk (where not all the study's prespecified outcomes had been reported; one or more reported primary outcomes were not prespecified outcomes of interest and were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk;
unclear risk
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Appendix 3. GRADE
GRADE considers that evidence from randomised controlled trials initially is "high" quality but that assessment may be downgraded based on consideration of any of five areas:
design (risk of bias);
consistency across studies;
directness of the evidence;
precision of estimates; and
presence of publication bias.
This results in an assessment of the quality of a body of evidence in one of four grades:
High: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Data and analyses
Comparison 1. Naloxone versus placebo or no drug.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Expired carbon dioxide output (mL/kg/min) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 15 minutes | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [0.15, 2.45] |
| 1.2 At 30 minutes | 3 | 108 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [0.39, 1.40] |
| 1.3 At 90 minutes | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐0.17, 1.17] |
| 1.4 At 4 hours | 2 | 58 | Mean Difference (IV, Fixed, 95% CI) | 0.86 [0.12, 1.59] |
| 2 Alveolar carbon dioxide tension (kPa) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At 10 minutes | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.01, ‐0.19] |
| 2.2 At 30 minutes | 3 | 102 | Mean Difference (IV, Fixed, 95% CI) | ‐0.87 [‐1.16, ‐0.59] |
| 2.3 At about 35 minutes | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.90, 0.70] |
| 2.4 At 4 hours | 2 | 58 | Mean Difference (IV, Fixed, 95% CI) | ‐0.87 [‐1.25, ‐0.50] |
| 3 Alveolar ventilation (mL/kg/minute) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 At 10 minutes | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 13.0 [‐16.88, 42.88] |
| 3.2 At 15 minutes | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 16.0 [‐8.53, 40.53] |
| 3.3 At 30 minutes | 4 | 152 | Mean Difference (IV, Fixed, 95% CI) | 36.03 [21.92, 50.14] |
| 3.4 At 90 minutes | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 14.0 [‐6.30, 34.30] |
| 3.5 At 4 hours | 2 | 58 | Mean Difference (IV, Fixed, 95% CI) | 55.30 [33.92, 76.69] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bonta 1979.
| Methods | Randomised controlled trial | |
| Participants | 43 term newborn infants whose mothers had received routine narcotic analgesia within 6 hours of delivery. Infants delivered in breech presentation or by Caesarean section, and infants with Apgar score less than 6 at 1 minute, were excluded | |
| Interventions | 1. Intramuscular naloxone (0.02 mg/kg body weight): n = 22 2. Placebo (normal saline): n = 21 | |
| Outcomes | Apgar score at 5 minutes, capillary blood gas values at 1, 2 and 4 hours of life, neurobehavioural assessment at 1, 4, and 24 hours | |
| Notes | NICU, North America, late 1970s | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generated in pharmacy to produce sequentially numbered ampoules containing either naloxone or placebo |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered ampoules (unable to predict whether naloxone or placebo) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled study using sequentially numbered ampoules ‐ personnel unlikely to have been aware of infants' group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The testers were not aware of which infants received naloxone or placebo" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete ascertainment of outcomes |
| Selective reporting (reporting bias) | Unclear risk | No protocol (though no reason to suspect selective reporting) |
| Other bias | Low risk | No reason to suspect any other bias |
Brice 1979a.
| Methods | Randomised controlled trial | |
| Participants | 50 term newborn infants whose mothers had received intramuscular pethidine within 4 hours of delivery | |
| Interventions | 1. Naloxone administered via the umbilical vein (0.01 or 0.02 mg/kg body weight): n = 26 2. No drug: n = 24 |
|
| Outcomes | Time to sustained respiration, expired carbon dioxide output, and alveolar ventilation up to 24 hours of life, Brazelton Neonatal Behavioral Assessment Score and Scanlon Behavioral Score within the first 24 hours of life | |
| Notes | NICU, United Kingdom, late 1970s | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Divided at random", no further details reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessors blinded for Scanlon developmental assessment (but results not reported for this outcome), no further details reported on blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | High risk | "The results for the serial blood‐gas analyses, the Brazelton and Scanlon scores ... are not presented in full. These may be obtained from [the corresponding author] on request." |
| Other bias | Unclear risk | Study was supported by a grant from Winthrop Laboratories (pharmaceutical company that manufactured the intervention) |
Dick 1978a.
| Methods | Randomised controlled trial | |
| Participants | 40 newborn infants, of unspecified gestation, whose mothers had been given intravenous pethidine in labour | |
| Interventions | 1. Naloxone, via the umbilical vein immediately after birth (0.02 mg/kg): n = 10 2. Naloxone (0.03 mg/kg): n = 10 3. Naloxone (0.04 mg/kg): n = 10 4. No drug: n = 10 |
|
| Outcomes | Capillary blood gas pH and partial pressure of carbon dioxide and of oxygen at 1, 5, 10, 30, 60 and 120 minutes of life | |
| Notes | NICU, Germany, late 1970s | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomly distributed", no further details reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol (though no reason to suspect selective reporting) |
| Other bias | Low risk | No reason to suspect any other bias |
Dick 1978b.
| Methods | Randomised controlled trial | |
| Participants | 30 newborn infants, of unspecified gestation, whose mothers had been given intravenous pethidine in labour | |
| Interventions | 1. Naloxone, via the umbilical vein immediately after birth (either 0.04 mg/kg or 0.04 mg total): n = 20 2. Placebo: n = 10 | |
| Outcomes | Capillary blood gas pH, partial pressure of carbon dioxide and of oxygen, and calculated base excess at 1, 5, 10, 30, 60 and 120 minutes of life | |
| Notes | NICU, Germany, late 1970s | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomly distributed", no further details reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Placebo and the varying amounts of Naloxone were distributed in equal amounts and thus indistinguishable" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Examiner and follow‐up examiner were not informed about the agent or the dose" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol (though no reason to suspect selective reporting) |
| Other bias | Low risk | No reason to suspect any other bias |
Evans 1976.
| Methods | Randomised controlled trial | |
| Participants | 44 newborn infants, of gestation 38 to 42 weeks, delivered spontaneously or by forceps, whose mothers had been given pethidine in labour | |
| Interventions | 1. Naloxone administered via the umbilical vein at 1 minute of age (0.04 mg total): n = 20 2. No drug: n = 24 |
|
| Outcomes | Time to first breath, time to onset of sustained respiration, Apgar score at 5 minutes, alveolar carbon dioxide tension, alveolar ventilation, and ventilation rate at 10 minutes and 30 minutes of life | |
| Notes | NICU, Wales, mid 1970s | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomly allocated", no further details reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol (though no reason to suspect selective reporting) |
| Other bias | Low risk | No reason to suspect any other bias |
Gerhardt 1977.
| Methods | Randomised controlled trial | |
| Participants | 20 term newborn infants, born vaginally, whose mothers had received intravenous pethidine within 3 hours of delivery | |
| Interventions | 1. Intramuscular naloxone at 30 minutes of life (0.01 mg/kg): n = 14 2. Placebo: n = 10 |
|
| Outcomes | Respiratory rate, tidal volume, minute ventilation, end‐tidal carbon dioxide tension, and the ventilatory response to inhalation of 4% carbon dioxide | |
| Notes | NICU, North America, mid 1970s | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "By random number ... infants were selected", no further details reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo‐controlled study but unclear if personnel were aware of infants' allocation to groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Near‐complete follow‐up but 2 infants in each group were excluded post‐randomisation because "lung compliance values changed more than 25% between the two determinations" |
| Selective reporting (reporting bias) | Unclear risk | No protocol (though no reason to suspect selective reporting) |
| Other bias | Low risk | No reason to suspect any other bias |
Welles 1984.
| Methods | Randomised controlled trial | |
| Participants | 27 newborn infants, of gestation 38 to 42 weeks, whose mothers had received pethidine during labour. Infants with Apgar scores less than 8 at 1 minute, or less than 9 at 5 minutes were not eligible for inclusion | |
| Interventions | 1. Naloxone at about 1 hour of age (0.1 mg total, presumed intramuscularly, but this was not stated explicitly): n = 14 2. Placebo (normal saline): n = 13 |
|
| Outcomes | Brazelton Neonatal Behavioral Assessment Score at 12‐24 hours of life and after a further 48 hours, and the Broussard Neonatal Perception Inventory after the second Brazelton assessment | |
| Notes | NICU, Sweden, early 1980s | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol (though no reason to suspect selective reporting) |
| Other bias | Low risk | No reason to suspect any other bias |
Wiener 1977a.
| Methods | Randomised controlled trial | |
| Participants | 28 newborn infants, of gestation 38 to 42 weeks, whose mothers had been given pethidine in labour | |
| Interventions | 1. Naloxone administered via the umbilical vein at 1 minute of age (0.04 mg total): n = 10 2. Normal saline placebo: n = 18 |
|
| Outcomes | Alveolar carbon dioxide tension, carbon dioxide excretion, alveolar ventilation, feeding behaviour, and habituation to a sound‐specific stimulus up to 48 hours of life | |
| Notes | NICU, Wales, mid 1970s | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation in pharmacy |
| Allocation concealment (selection bias) | Low risk | Coded ampoules |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Naloxone or normal saline were "chosen blind at random" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Likely to have been blinded but not stated explicitly either way in the paper |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol (though no reason to suspect selective reporting) |
| Other bias | Unclear risk | The study was stopped early because the randomisation code was broken after 28 infants had been enrolled. |
Wiener 1977b.
| Methods | Randomised controlled trial | |
| Participants | 30 newborn infants, of gestation 38 to 42 weeks, whose mothers had been given pethidine in labour | |
| Interventions | 1. Intramuscular naloxone at 1 minute of age (0.2 mg total): n = 15 2. Intramuscular normal saline placebo: n = 15 |
|
| Outcomes | Alveolar carbon dioxide tension, carbon dioxide excretion, alveolar ventilation, feeding behaviour (mean sucking frequencies and pressures, and mean milk consumption), and habituation to a sound‐specific stimulus up to 48 hours of life | |
| Notes | NICU, Wales, mid 1970s | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation in pharmacy |
| Allocation concealment (selection bias) | Low risk | Coded ampoules |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Naloxone or normal saline were "chosen blind at random" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Likely to have been blinded but not stated explicitly either way in the paper |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol (though no reason to suspect selective reporting) |
| Other bias | Low risk | No reason to suspect other bias |
NICU: Neonatal intensive care unit
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brice 1979b | This report of pharmacokinetic data in infants who received either intravenous or intramuscular naloxone was unlikely to be a randomised comparison |
| Martin 1972 | This report was unlikely to be a randomised controlled trial |
Differences between protocol and review
For the 2018 update, we made the following changes:
Neurodevelopment promoted to primary outcome because ILCOR has ranked this as a "critical" outcome across the board in the 2015 consensus statement (Perlman 2015).
We changed the title of the review from "opiate" to "opioid", as a broader term. The original protocol title was "Naloxone for narcotic‐exposed newborn infants".
We added the methodology and plan for 'Summary of findings' tables and GRADE recommendations, which were not included in the original protocol or the previously published review.
Contributions of authors
Thirimon Moe‐Byrne and Jennifer Brown searched and screened the studies for inclusion, assessed the methodological quality of the trials, and extracted and entered the relevant information and data from each included study independently. All authors completed the final review.
Sources of support
Internal sources
Centre for Reviews and Dissemination, University of York, UK.
External sources
-
National Institute for Health Research (NIHR), UK.
This report is independent research funded by a UK NIHR Cochrane Programme Grant (16/114/03). The views expressed in this publication are those of the review authors and are not necessarily those of the National Health Service, the NIHR, or the UK Department of Health.
-
Vermont Oxford Network, USA.
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
Declarations of interest
None.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bonta 1979 {published data only}
- Bonta BW, Gagliardi JV, Williams V, Warshaw JB. Naloxone reversal of mild neurobehavioral depression in normal newborn infants after routine obstetric analgesia. Journal of Pediatrics 1979;94(1):102‐5. [PUBMED: 363995] [DOI] [PubMed] [Google Scholar]
Brice 1979a {published data only}
- Brice JE, Moreland TA, Walker CH. Effects of pethidine and its antagonists on the newborn. Archives of Disease in Childhood 1979;54(5):356‐61. [PUBMED: 383023] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dick 1978a {published data only}
- Dick W, Knoche E, Traub E. Clinical investigations of the influence of various naloxone doses on the newborn. Journal of Perinatal Medicine 1978;6(2):95‐110. [PUBMED: 29088] [DOI] [PubMed] [Google Scholar]
Dick 1978b {published data only}
- Dick W, Knoche E, Traub E. Clinical investigations of the influence of various naloxone doses on the newborn. Journal of Perinatal Medicine 1978;6(2):95‐110. [PUBMED: 29088] [DOI] [PubMed] [Google Scholar]
Evans 1976 {published data only}
- Evans JM. The effect of naloxone on the early respiratory depressant effect of maternal pethidine analgesia. 4th European Congress of Anaesthesiology; 1974 Sep 5‐11; Madrid (Spain). New York (NY): Excerpta Medica, 1974.
- Evans JM, Hogg MI, Rosen M. Reversal of narcotic depression in the neonate by naloxone. BMJ 1976;2(6044):1098‐100. [PUBMED: 990784] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JM, Hogg MI, Rosen M. The effect of naloxone on the depression of the early respiratory activity of neonates produced by maternal pethidine analgesia. In: Arias A editor(s). Recent Progress in Anaesthesiology and Resuscitation: IV European Congress of Anaesthesiology. Amsterdam: Excerpta Medica, 1975:72. [Google Scholar]
Gerhardt 1977 {published data only}
- Gerhardt T, Bancalari E, Cohen H, Rocha LF. Use of naloxone to reverse narcotic respiratory depression in the newborn infant. Journal of Pediatrics 1977;90(6):1009‐12. [PUBMED: 323443] [DOI] [PubMed] [Google Scholar]
- Gerhardt T, Bancalari E, Cohen H, Rocha LF, Holsinger K. Reversal of narcotic respiratory depression in the newborn with naloxone. American Society of Anesthesiologists Annual Meeting. Chicago, 1975.
Welles 1984 {published data only}
- Welles B, Belfrage P, Chateau P. Effects of naloxone on newborn infant behavior after maternal analgesia with pethidine during labor. Acta Obstetricia et Gynecologica Scandinavica 1984;63(7):617‐9. [PUBMED: 6516811] [DOI] [PubMed] [Google Scholar]
Wiener 1977a {published data only}
- Wiener PC, Hogg MI, Rosen M. Effects of naloxone on pethidine‐induced neonatal depression. Part 1 ‐ Intravenous naloxone. BMJ 1977;2(6081):228‐9. [PUBMED: 328108] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiener 1977b {published data only}
- Wiener PC, Hogg MI, Rosen M. Effects of naloxone on pethidine‐induced neonatal depression. Part 2. Intramuscular naloxone. BMJ 1977;2(6081):229‐31. [PUBMED: 884446] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener PC, Hogg MI, Rosen M. Neonatal respiration, feeding and neurobehavioural state. Effects of intrapartum bupivacaine, pethidine and pethidine reversed by naloxone. Anaesthesia 1979;34(10):996‐1004. [PUBMED: 395854] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Brice 1979b {published data only}
- Brice JE, Moreland TA, Parija AC, Walker CH. Plasma naloxone levels in the newborn after intravenous or intramuscular administration. British Journal of Clinical Pharmacology 1979;8(4):412P‐3P. [PUBMED: 508546] [PubMed] [Google Scholar]
Martin 1972 {published data only}
- Martin K, Knapstein PG, Melchert F, Schafer H, Tietze KW. Clinical experiences with the opiate antagonist naloxone in newborns [Klinische Erfahrungen mit dem Opiat‐Antagonisten naloxone beim Neugeborenen]. Tagung der Mittelrheinischen gesellschaft fur Geburtshilfe und Gynakologie 1972;143:10‐11. [Google Scholar]
Additional references
AAP 1980
- American Academy of Pediatrics Committee on Drugs. Naloxone use in newborns. Pediatrics 1980;65(3):667‐9. [PubMed] [Google Scholar]
AAP 1990
- American Academy of Pediatrics Committee on Drugs. Naloxone dosage and route of administration for infants and children: addendum to emergency drug doses for infants and children. Pediatrics 1990;86(3):484‐5. [PUBMED: 2388800] [PubMed] [Google Scholar]
AAP 1998
- American Academy of Pediatrics Committee on Drugs. Neonatal drug withdrawal. Pediatrics 1998;101(6):1079‐88. [PUBMED: 9614425] [PubMed] [Google Scholar]
Burchell 2016
- Burchell T, Coster S, Norman I. The effect of intrapartum pethidine on breastfeeding: a scoping review. Evidence Based Midwifery 2016;14(2):49‐56. [Google Scholar]
De Castro 1993
- Castro RM, Cabral‐Filho JE, Costa JA, Costa FB, Gallindo MA, Hecksher CA. Neonatal treatment with naloxone causes permanent hyperalgesia in rats. Brazilian Journal of Medical & Biological Research 1993;26(7):747‐51. [PUBMED: 8268823] [PubMed] [Google Scholar]
Gibbs 1989
- Gibbs J, Newson T, Williams J, Davidson DC. Naloxone hazard in infant of opioid abuser. Lancet 1989;2(8655):159‐60. [PUBMED: 2567922] [DOI] [PubMed] [Google Scholar]
Gill 2007
- Gill AW, Colvin J. Use of naloxone during neonatal resuscitation in Australia: compliance with published guidelines. Journal of Paediatrics and Child Health 2007;43(12):795‐8. [DOI: 10.1111/j.1440-1754.2007.01194.x; PUBMED: 17803674] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 15 March 2018. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Herschel 2000
- Herschel M, Khoshnood B, Lass NA. Role of naloxone in newborn resuscitation. Pediatrics 2000;106(4):831‐4. [PUBMED: 11015529] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Higgins 2017
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook.
James 2012
- James JN, Prakash KS, Ponniah M. Awareness and attitudes towards labour pain and labour pain relief of urban women attending a private antenatal clinic in Chennai, India. Indian Journal of Anaesthesia 2012;56(2):195‐8. [DOI: 10.4103/0019-5049.96331; PUBMED: 22701219] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2012
- Jones L, Othman M, Dowswell T, Alfirevic Z, Gates S, Newburn M, et al. Pain management for women in labour: an overview of systematic reviews. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD009234.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kattwinkel 2010
- Kattwinkel J, Perlman JM, Aziz K, Colby C, Fairchild K, Gallagher J, et al. Part 15: neonatal resuscitation: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010;122(18 Suppl 3):S909‐19: Erratum in: Circulation 2011;124(15):e406. [DOI: 10.1161/CIRCULATIONAHA.110.971119; PUBMED: 20956231] [DOI] [PubMed] [Google Scholar]
Kumar 2003
- Kumar M, Paes B. Epidural opioid analgesia and neonatal respiratory depression. Journal of Perinatology 2003;23(5):425‐7. [DOI: 10.1038/sj.jp.7210905; PUBMED: 12847541] [DOI] [PubMed] [Google Scholar]
Lefebvre 2008
- Lefebvre C, Eisinga A, McDonald S, Paul N. Enhancing access to reports of randomized trials published world‐wide ‐ the contribution of EMBASE records to the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library. Emerging Themes in Epidemiology 2008;5:13. [DOI: 10.1186/1742-7622-5-13] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Lutz 2016
- Lutz TL, Elliott EJ, Jeffery HE. Sudden unexplained early neonatal death or collapse: a national surveillance study. Pediatric Research 2016;80(4):493‐8. [PUBMED: 27384403] [DOI] [PubMed] [Google Scholar]
Maas 1990
- Maas U, Kattner E, Weingart‐Jesse B, Schafer A, Obladen, M. Infrequent neonatal opiate withdrawal following maternal methadone detoxification during pregnancy. Journal of Perinatal Medicine 1990;18(2):111‐8. [PUBMED: 2366131] [DOI] [PubMed] [Google Scholar]
Mercer 2007
- Mercer JS, Erickson‐Owens DA, Graves B, Haley MM. Evidence‐based practices for the fetal to newborn transition. Journal of Midwifery & Women's Health 2007;52(3):262‐72. [DOI: 10.1016/j.jmwh.2007.01.005; PUBMED: 17467593] [DOI] [PubMed] [Google Scholar]
NICE 2017
- National Institute for Health and Care Excellence. Intrapartum care for healthy women and babies: Clinical Guideline CG190. www.nice.org.uk/guidance/cg190 (accessed 25 July 2018).
Niermeyer 2001
- Niermeyer S, Reempts P, Kattwinkel J, Wiswell T, Burchfield D, Saugstad OD, et al. International Liaison Committee on Resuscitation. Resuscitation of newborns. Annals of Emergency Medicine 2001;37(4 Suppl):S110‐25. [PUBMED: 11290975] [DOI] [PubMed] [Google Scholar]
Nissen 1995
- Nissen E, Lilja G, Matthiesen AS, Ransjo‐Arvidsson AB, Uvnas‐Moberg K, Widstrom AM. Effects of maternal pethidine on infants' developing breast feeding behaviour. Acta Paediatrica 1995;84(2):140‐5. [PUBMED: 7756797] [DOI] [PubMed] [Google Scholar]
Ogboli‐Nwasor 2014
- Ogboli‐Nwasor EO, Adaji SE. Petween pain and pleasure: pregnant women's knowledge and preferences for pain relief in labor, a pilot study from Zaria, Northern Nigeria. Saudi Journal of Anaesthesia 2014;8(Suppl 1):S20‐4. [DOI: 10.4103/1658-354X.144059; PUBMED: 25538515] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perlman 2010
- Perlman JM, Wyllie J, Kattwinkel J, Atkins DL, Chameides L, Goldsmith JP, et al. Neonatal Resuscitation Chapter Collaborators. Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Pediatrics 2010;126(5):e1319‐44. [DOI: 10.1542/peds.2010-2972B; PUBMED: 20956431] [DOI] [PubMed] [Google Scholar]
Perlman 2015
- Perlman JM, Wyllie J, Kattwinkel J, Wyckhoff MH, Aziz K, Guinsburg R, et al. Neonatal Resuscitation Chapter Collaborators. Part 7: Neonatal Resuscitation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 2015;132(16 Suppl 1):S204‐41. [DOI: 10.1161/CIR.0000000000000276; PUBMED: 26472855] [DOI] [PubMed] [Google Scholar]
Ransjo‐Arvidson 2001
- Ransjo‐Arvidson AB, Matthiesen AS, Lilja G, Nissen E, Widstrom AM, Uvnas‐Moberg K. Maternal analgesia during labor disturbs newborn behavior: effects on breastfeeding, temperature, and crying. Birth 2001;28(1):5‐12. [PUBMED: 11264622] [DOI] [PubMed] [Google Scholar]
Redshaw 2007
- Redshaw M, Rowe R, Hockley C, Brocklehurst P. Recorded delivery: a national survey of women's experience of maternity care 2006. www.npeu.ox.ac.uk/downloads/files/reports/Maternity‐Survey‐Report.pdf (accessed 24 July 2018).
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Reynolds 2010
- Reynolds F. The effects of maternal labour analgesia on the fetus. Best Practice & Research. Clinical Obstetrics & Gynaecology 2010;24(3):289‐302. [DOI: 10.1016/j.bpobgyn.2009.11.003; PUBMED: 20005180] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). gdt.guidelinedevelopment.org/app/handbook/handbook.html (accessed prior to 29 September 2018).
Smotherman 1992
- Smotherman WP, Robinson SR. Prenatal experience with milk: fetal behavior and endogenous opioid systems. Neuroscience & Biobehavioral Reviews 1992;16(3):351‐64. [DOI] [PubMed] [Google Scholar]
Szeto 1995
- Szeto HH, Soong Y, Wu DL, Cheng PY. Opioid modulation of fetal glucose homeostasis: role of receptor subtypes. Journal of Pharmacology and Experimental Therapeutics 1995;275(1):334‐9. [PUBMED: 7562568] [PubMed] [Google Scholar]
Ullman 2010
- Ullman R, Smith LA, Burns E, Mori R, Dowswell T. Parenteral opioids for maternal pain management in labour. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD007396.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
McGuire 2002
- McGuire W, Fowlie PW. Naloxone for narcotic‐exposed newborn infants. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD003483] [DOI] [PubMed] [Google Scholar]
Moe‐Byrne 2013
- Moe‐Byrne T, Brown JV, McGuire W. Naloxone for opiate‐exposed newborn infants. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD003483.pub2; PUBMED: 23450541] [DOI] [PubMed] [Google Scholar]


