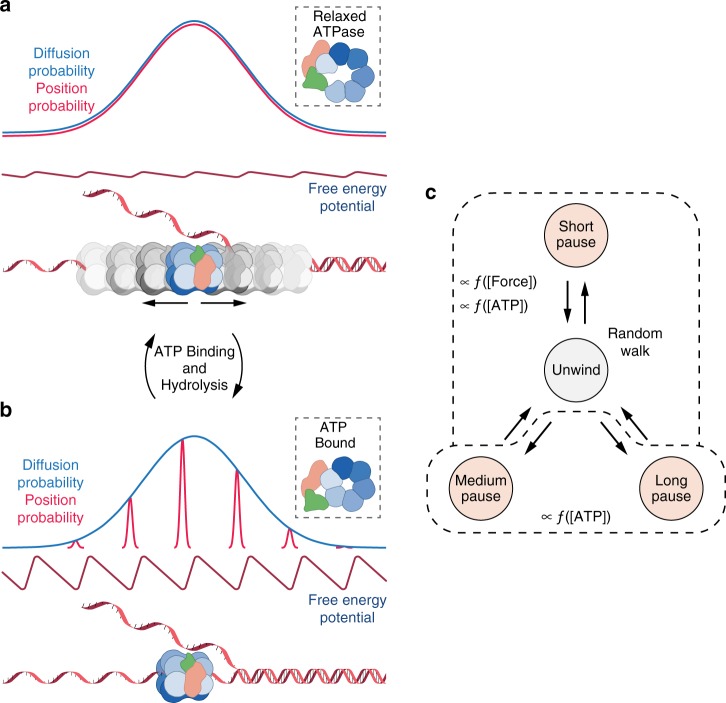
Fig. 5.
Proposed Brownian ratchet mechanism and most likely kinetic scheme. a In the weakly bound state, open conformer where the Mcm2,5 gate is open (dashed box). CMG can undergo unbiased diffusion (random walk) along the DNA due to a weak free energy potential. b Upon ATP binding/hydrolysis CMG undergoes an allosteric structural change that alters the affinity for DNA, increasing the free energy potential of interaction with the DNA. In this state, CMG remains at the bottom of the potential well, fixed in position. The asymmetry in the potential means that upon cycling between these two states through ATP hydrolysis CMG is more likely to be in the (n + 1)th potential well representing the next base along, giving rise to a bias towards the unwinding direction. c The proposed kinetic scheme for DNA unwinding by CMG. The enzyme is in an active state, unwinding via a Brownian ratchet mechanism but may enter one of three pauses. The kinetics governing theses process are dependent on force applied to DNA and the ATP concentration