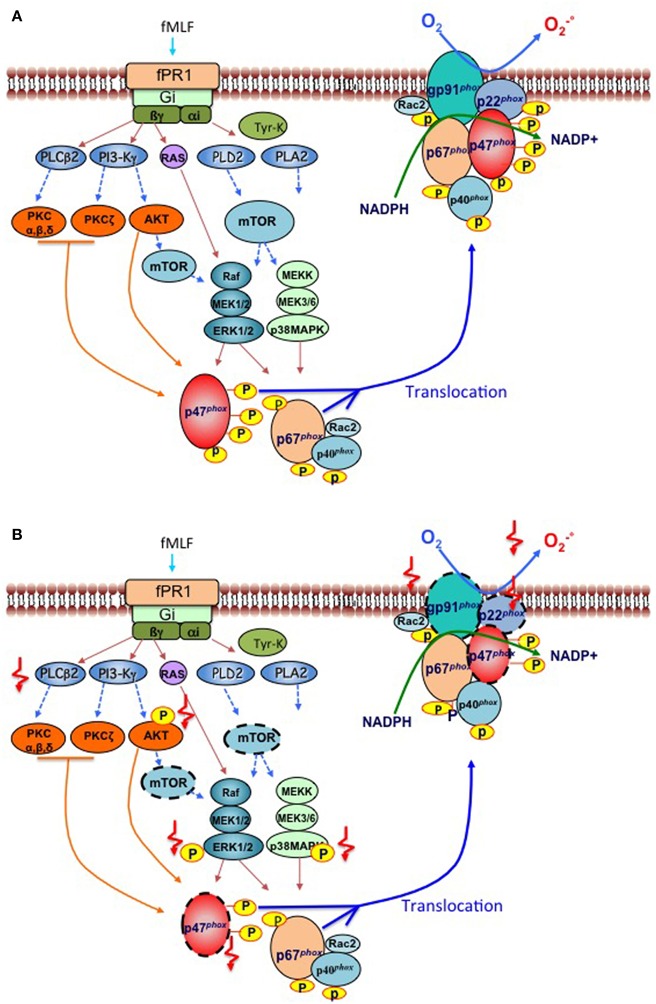
Figure 1.
Signaling pathways involved in phosphorylation and activation of the NADPH oxidase induced by bacterial peptides in human neutrophils from respectively “healthy subjects” and “cirrhotic patients”. (A) Healthy subjects. The binding of the bacterial formylated peptide fMet-leu-Phe (fMLF) to its Gi-protein-coupled receptor fPR1, triggers the activation of various major early signaling effectors such as phospholipase C (PLCß2), Phospholipase D (PLD2), Phospholipase A (PLA2), Phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3-Kγ), tyrosine kinases, and the small G-protein Ras. Second messengers produced by phospholipases stimulate various protein kinases, such as protein kinase C (PKC) isoforms, protein kinase B (AKT1/2), mammalian target of rapamycin (mTOR), which in turn activates two major families of mitogen-activated protein kinases (MAPKs), including ERK1/2 and p38-MAPK. PKCs and MAPKs phosphorylate cytosolic components of the NADPH oxidase (p47phox, p67phox, p40phox) which allows their translocation to the plasma membrane, together with the small G protein Rac2, to activate a cytochrome b, constituted with the gp91phox (also known as NOX2) and its partner p22phox. The activated NOX2 reduces oxygen to superoxide () at the expense of NADPH. (B) Patients with cirrhosis. Two types of deficiencies have been identified in neutrophils of cirrhotic patients stimulated in vitro by bacterial peptides; those which decrease the activation/phosphorylation of signaling effectors such as AKT, MAP-Kinase ERK1/2, and p38-MAP kinase, p47phox; and PLCβ2 activity (indicated by  ) and those which decrease the expression of protein effectors (indicated by a dashed outline,
) and those which decrease the expression of protein effectors (indicated by a dashed outline,  ) especially for mTOR, gp91phox, p22phox, and p47phox. These alterations lead to a deficient production of superoxide anion by gp91phox.
) especially for mTOR, gp91phox, p22phox, and p47phox. These alterations lead to a deficient production of superoxide anion by gp91phox.