Abstract
Germin-like proteins (GLPs) are water-soluble plant glycoproteins belonging to the cupin superfamily. The important role of GLPs in plant responses against various abiotic and biotic stresses, especially pathogens, is well validated. However, little is known about cotton GLPs in relation to fungal pathogens. Here, a novel GLP gene was isolated from Gossypium hirsutum and designated as GhABP19. The expression of GhABP19 was upregulated in cotton plants inoculated with Verticillium dahliae and Fusarium oxysporum and in response to treatment with jasmonic acid (JA) but was suppressed in response to salicylic acid treatment. A relatively small transient increase in GhABP19 was seen in H2O2 treated samples. The three-dimensional structure prediction of the GhABP19 protein indicated that the protein has three histidine and one glutamate residues responsible for metal ion binding and superoxide dismutase (SOD) activity. Purified recombinant GhABP19 exhibits SOD activity and could inhibit growth of V. dahliae, F. oxysporum, Rhizoctonia solani, Botrytis cinerea, and Valsa mali in vitro. To further verify the role of GhABP19 in fungal resistance, GhABP19-overexpressing Arabidopsis plants and GhABP19-silenced cotton plants were developed. GhABP19-transgenic Arabidopsis lines showed much stronger resistance to V. dahliae and F. oxysporum infection than control (empty vector) plants did. On the contrary, silencing of GhABP19 in cotton conferred enhanced susceptibility to fungal pathogens, which resulted in necrosis and wilt on leaves and vascular discoloration in GhABP19-silenced cotton plants. The H2O2 content and endogenous SOD activity were affected by GhABP19 expression levels in Arabidopsis and cotton plants after inoculation with V. dahliae and F. oxysporum, respectively. Furthermore, GhABP19 overexpression or silencing resulted in activation or suppression of JA-mediated signaling, respectively. Thus, GhABP19 plays important roles in the regulation of resistance to verticillium and fusarium wilt in plants. These modulatory roles were exerted by its SOD activity and ability to activate the JA pathway. All results suggest that GhABP19 was involved in plant disease resistance.
Keywords: germin-like protein, Gossypium hirsutum, Verticillium dahliae, Fusarium oxysporum, superoxide dismutase, disease resistance
Introduction
Germin-like proteins (GLPs) are diverse and ubiquitous plant glycoproteins, ordinarily found in various terrestrial plants (Rebeccam et al., 2009). GLPs belong to the cupins, a functionally diverse superfamily of proteins that contains a conserved β-barrel core, which is involved in manganese ion binding (Dunwell et al., 2008). Previous studies have indicated that GLPs are stable in heat, extreme pH, and detergent treatments (Woo et al., 2000), and they can function as various enzymes, such as superoxide dismutase (SOD; Banerjee and Maiti, 2010; Rietz et al., 2012; Zhang et al., 2017), oxalate oxidase (OXO; Sakamoto et al., 2015), and ADP glucose pyrophosphatase/phosphodiesterase (Fan et al., 2005), polyphenol oxidase (Cheng et al., 2014), as well as rhicadhesin receptors (Swart et al., 1994).
Based on plant-microbe interactions, GLPs are considered a pathogenesis-related (PR) protein 16 family (Park et al., 2004). Many GLPs are localized in the cell wall and function as cofactors for cell wall reinforcement by facilitating the cross-linking of plant cell wall components during the formation of papillae, which renders them resistant to infection; this process involves the generation of H2O2 because of their SOD or OXO activity (Wei et al., 1998; Liu Q. et al., 2016; Zhang et al., 2017). SOD is responsible for the dismutation of superoxide to H2O2 and O2 and the protection of cells from oxidative burst (Sultana et al., 2016). H2O2 can act as a signaling molecule for the induction of systemic acquired resistance of non-inoculated tissues (Tran et al., 2014). In addition, H2O2 can initiate salicylic acid (SA) and/or jasmonic acid (JA) signaling pathways, leading to the synthesis of pathogenesis-related protein synthesis and plant defenses, respectively (Leon et al., 1995).
An increasing amount of evidence has shown that GLPs are a crucial component of plant basal host resistance and can be upregulated and/or activated by pathogen infection or by the application of disease resistance–associated chemicals such as H2O2, SA, and ethylene (Lou and Baldwin, 2006; Zimmermann, 2006; Godfrey et al., 2007; Himmelbach et al., 2010). The heterologous expression of GLPs or gene silencing of endogenous GLPs have also provided evidence for their involvement in defense against fungal pathogens. For example, the transient overexpression of HvGER4 and HvGER5 or silencing of HvGER3 protect barley epidermal cells from Blumeria graminis infection (Zimmermann, 2006). Moreover, the heterologous expression of BvGLP-1 from sugar beet (Beta vulgaris) increased the resistance of transgenic Arabidopsis thaliana against Verticillium longisporum and Rhizoctonia solani infection (Knecht et al., 2010). In Lilium regale, the LrGLP1 gene could be induced by exogenous ethylene and the incompatible interaction between L. regale and Fusarium oxysporum f. sp. lilii; the LrGLP1 transgenic tobacco lines showed considerably stronger resistance to F. oxysporum f. sp. lilii infection than did the wild-type plants (Zhang et al., 2017). Furthermore, the OsRGLP1 gene was highly expressed in Medicago truncatula transformed lines and provided protection against F. oxysporum (Sultana et al., 2016).
Verticillium and fusarium wilt, caused by Verticillium dahliae and F. oxysporum, respectively, are two important fungal diseases of cotton that affect the vascular tissues (Ma et al., 1997; Zambounis et al., 2008). It is extremely difficult to control verticillium wilt in cotton, as its hyphae reside in the woody vascular tissues and thus cannot be destroyed by fungicides. In addition, V. dahliae survives in soil for many years because of the production of microsclerotia (Fradin and Thomma, 2006). F. oxysporum is widespread and pathogenic to various plant species. It is able to grow and survive over long periods on organic matter in soil and in the rhizosphere of many plants. This fungus penetrates the roots or invades the vascular system of host plants, causing either root-rot or tracheomycosis (Fravel et al., 2003). A series of resistance-related genes from cotton have been studied to uncover the resistance mechanisms of cotton against V. dahliae and F. oxysporum (Liu et al., 2017b; Li et al., 2018; Wang et al., 2018). However, data on the functions of cotton GLPs in fungal defense are still poorly understood. Therefore, assessing the biochemical properties of GhABP19 and identifying its functions in cotton disease resistance is necessary.
Previous studies have shown that two homologous auxin-binding proteins, PpABP19 and PpABP20, isolated from shoot apices of Prunus persica, are highly homologous to families of germin and GLPs and belong to the GLP family (Ohmiya et al., 1998; Ohmiya, 2002). In the present study, a novel ABP19 gene was isolated from Gossypium hirsutum. The expression patterns of GhABP19 that can be induced by pathogen infection, abiotic stresses, and phytohormones were characterized in cotton. We generated a recombinant GhABP19 protein by expressing it in Escherichia coli and evaluated its role in the defense against fungal infections and its SOD activity. GhABP19 is involved in the defense against V. dahliae and F. oxysporum infection; the results were examined by generating GhABP19-overexpressing Arabidopsis plants and GhABP19-silenced cotton plants through virus-induced gene silencing (VIGS) assays. Further, we showed that GhABP19 is involved in the JA-mediated defense response.
Materials and Methods
Plant Materials and Fungal Strains
The resistant cotton cultivar Zhongzhimian 2 (original strain GK44) and the susceptible cultivar Xinluzao 33 provided by the Cotton Research Institute were grown under standard conditions, and their seedlings were used in this study. The seeds of A. thaliana (ecotype Columbia) were sterilized, rinsed and then sowed on Murashige-Skoog (MS) culture medium. After 4°C verbalization for 3 days, seeds were cultured in chamber (22°C/18°C, 16 h light/8 h dark) and 10-days-old seedlings were transplanted into soil. Virulent strains of V. dahliae (strain Vd991) and F. oxysporum (strain AYF-1) were grown in the potato dextrose agar (PDA) at 25°C for a week. Colonies were then transferred to 500-mL Erlenmeyer flasks containing 100 mL Czapek’s liquid medium and grown at 25°C for 7 days. Concentrated spore solutions were then prepared using 107-conidia/mL suspensions.
Cloning of ABP19 cDNA
Total RNA of Zhongzhimian 2 cotton cultivar complete stool (grew under standard conditions) was extracted using a commercially available kit (Promega, Madison, WI, United States). Polyadenylated mRNA was separated with a PolyATract mRNA Isolation System (Promega, Madison, WI, United States). Subsequently, a cDNA library was generated by inserting fragments in a λZAP-II vector as the specifications of cDNA Library Construction Kit (Merck, Germany). The library was propagated on 140 mm plates to obtain about 105 plaques (Wang et al., 2011a). The conserved region of AtGLP3a isolated from Arabidopsis (Staiger et al., 1999), was labeled with 32P-dUTP and used as probe for positive plaques by colony in situ hybridization. A positive plaque was obtained after three rounds, and the fragment was subcloned into pBlueScript II SK (+) through in vivo excision, following the protocol provided by manufacturer (Stratagene, United States).
Bioinformatic Analyses of GhABP19
The open reading frame (ORF) of GhABP19 was identified using the program ORF Finder at the NCBI web-site1. The prediction of the putative signal peptide sequence was done at the SignalP 4.1 server2. Theoretical molecular mass and isoelectric point (pI) were assessed through the ProtParam website3. Post-translationa modifications as N-acetylation and phosphorylation were predicted through NetAcet 1.0 and NetPhos 2.0 server, respectively (Blom et al., 2004; Kiemer et al., 2005). Multiple sequence alignment was conducted with Clustal Omega4 under default settings. The phylogenetic tree was performed by MEGA 7.0 software using the neighbor-joining method (Kumar et al., 2016). The Genbank accession numbers of germin and germin-like protein sequences have been presented in Supplementary Table S1.
Expression Analysis of the GhABP19
For pathogen treatment, roots of 2-week-old cotton seedlings were inoculated with V. dahliae and F. oxysporum conidial suspension (both of 107-conidia/mL) for 5 min, respectively, then transplanted into pots with fresh soil. For abiotic stresses and hormone treatments, cotton seedlings of true leaf stage were uprooted from soil and replanted in Hoagland medium, which contained 100 mM H2O2, 1 mM SA and 100 μM JA, respectively. The leaf tissues from cotton plants were harvested at an appropriate time for RNA extraction. Total RNA was isolated using an EASYspin RNA Extraction Kit (Biomed, China) from cotton tissues. First-strand cDNA was synthesized by Fast Quant cDNA Reverse Kit (TIANGEN BIOTECH CO., LTD.), diluted, and used as template for real-time quantitative reverse transcription-polymerase chain reaction (qRT-PCR). GhABP19 was amplified by primer sequences 5′-CAACGCAGCCGACTTCTGTG-3′ and 5′-CCCAGGGTGTGTATGATAGG-3′. The endogenous gene UBQ7 (DQ116441) from cotton was used as an internal standard, and can be detected using the primers 5′-GACCCTTCCTCTATATAAG-3′ and 5′-GGACAACTCCATGAAAAG-3′. Reactions were prepared in 20 μL with SYBR Premix Ex Taq (Tli RNaseH Plus; Takara, Shiga, Japan) and amplification was performed on an ABI 7500 thermocycler (Applied Biosystems, Foster city, CA, United States). qRT-PCR assays were carried out in three independent biological samples per treatment and three technical replicates per samples. Expression was calculated with 2-ΔΔCT method and data were analyzed in Origin 8.
Expression and Purification of Recombinant GhABP19 Protein
GhABP19 was amplified using primers, which included restriction sites for Nde I and Hind III enzymes in the forward and reverse ones, respectively. The sequence of these primers (restriction sites are underlined) were as follows: (Forward)5′-GGACCATATGATGGACATACCTTCAAGGAC-3′, (Reverse) 5′-ACCCAAGCTT ACCAGTTCCTCCAAGAACA-3′. The pET-22b(+) protein expression vector (Novagen) was also digested with Nde I and Hind III and then ligated with the GhABP19 fragment by using T4 ligase (Promega) for overnight at 16°C; the resulting construct was named as pET-22b(+)-GhABP19. Next, the construct was transformed into E. coli BL21 (DE3), and single colonies were cultured at 37°C in Luria–Bertani broth supplemented with 100 μg/mL ampicillin. The cultures were scaled up by inoculating precultures at 1% into fresh media. At OD 0.6–0.7, they were immediately induced with 0.1 mM IPTG and cultured for another 4 h at 37°C with oscillation. Samples were harvested by centrifugation for 10 min at 10,000 ×g and then resuspended in 20 mL phosphate buffered saline (PBS, pH 7.4). Cells were disrupted using a sonicator (Ultrasonic processor), and then centrifuged at 10,000 ×g for 10 min. Inclusion fraction was separated and resuspended in PBS. Protein was verified using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and GhABP19 purification was performed using 6 × His-Tagged Protein Purification Kit (CW BIO) following manufacturer’s recommendations.
Antifungal Activity Assay of Purified GhABP19 Protein
The fungal species V. dahliae, F. oxysporum, R. solani, B. cinerea, and V. mali were grown in PDA plates at 25°C. Five different concentrations (35, 70, 140, 280, and 560 μg/mL) of GhABP19 were added in PDA plates at 25°C, then a small amount of mycelia (0.5 cm in diameter) was placed on the surface of the agar. The half maximal inhibitory concentration (IC50) of pure protein was determined as previous described (Wang et al., 2011b). Data were collected from three independent replicates.
Expression Vector Constructs and Generation of Arabidopsis Transgenic Plants
Plant expression vector Super-pCAMBIA1300 was used in the present study. The ORF of GhABP19 was amplified using the forward primer 5′-CTAGTCTAGAATGGACATACCTTCAAGGAC-3′ and reverse primer 5′-AAGGACTAGTACCAGTTCCTCCAAGAACA-3′, which restriction sites Xba I and Spe I were, respectively, added (restriction sites are underlined). Then, the gene was ligated into the digested Super-pCAMBIA1300 vector with the same two restriction endonucleases for fusion with C-terminal green fluorescent protein gene (GhABP19-GFP). The vector expressed GhABP19 under control of the CaMV35S promoter and carried the hygromycin phosphotransferase (htp II) resistance gene as a selectable marker for the selection of transformed plants (Supplementary Figure S1A). The plasmid was introduced into Agrobacterium tumefaciens strain GV3101 by freeze–thaw method. Arabidopsis plants were transformed by the floral dip method via Agrobacterium-mediated transformation procedure (Clough and Bent, 1998), control plants were transformed with Super1300-GFP empty vector plasmid.
Infection Assay of Arabidopsis Plants With Fungal Conidia in Soil
Antifungal activities induced by GhABP19 in Arabidopsis plants after V. dahliae and F. oxysporum infection were assessed using the method described before (Stoilova and Chavdarov, 2006) with slight modification. Seedlings of Arabidopsis plants were cultivated in soil in small pots for 4 weeks under normal growth condition. When the spore concentrations of V. dahliae and F. oxysporum reached 107 conidia/mL, the susceptibility of Arabidopsis to these isolates was determined by dipping the roots of 4-week-old GhABP19-transgenic and control (empty vector) plants in fungal culture for 5 min after wounding four to five roots on each plant by trimming before planting. The same number of plants injured and dipped in sterilized water served as non-infected controls. The symptoms of V. dahliae and F. oxysporum infection on Arabidopsis plant leaves in the form of yellowing, wilting, and drying were recorded for 30 and 49 days, respectively. Severity of infection on the plants from mild to severe was recorded on a scale of 1–5, indicating no symptoms, greenish yellow leaves, yellow leaves, mildly wilted leaves, and wilted and completely dried leaves, respectively. The numbers of leaves in each grade were recorded. Percent disease index (PDI) was calculated using the following formula:
where S1–S5 stand for the number of leaves in each scale. Morphological and growth data on infected transgenic plants were compared with those of infected control plants. Data were collected from three independent replicates (n ≥ 30), and similar results were obtained.
Roots Staining Assays and Light Microscopy Observations
To monitor fungal hyphae on or in Arabidopsis roots, seedlings were removed from agar plates, roots of control and transgenic Arabidopsis plants were washed thoroughly in running tap water, cut into 1-cm pieces and treated overnight with 10% KOH solution at room temperature. Thereafter, the root pieces were washed 3–5 times with sterilized distilled water and treated with 1% HCl for 3–4 min before staining with 0.05% trypan blue in lactophenol. The stained root segments were examined microscopically. The assays were repeated three times (n ≥ 20), and similar results were obtained.
Virus-Induced Gene Silencing (VIGS)
Silenced fragments of GhCLA1 (cloroplastos alterados 1) and GhABP19 were cloned from Zhongzhimian 2 cotton cultivar cDNA. In detail, the GhCLA1-silenced fragment (500 bp) was cloned using the primers 5′-GGAATTCCACAACATCGATGATTTAG-3′, and 5′-GGGGTACCATGATGAGTAGATTGCAC-3′, according to previous study (Gao et al., 2011). The GhABP19-silenced fragment (457 bp) was amplified using the primers 5′-CGACGACAAGACCGTGACCATGAAATGACTTCGTTTACTCCGGCC-3′ and 5′-GAGGAGAAGAGCCGTCATTAGCTTCTTAATCTGAGCAGCATCCAG-3′. In a subsequent step, both of the silenced fragments were inserted into the TRV:00 vector by ligation-independent cloning to generate the TRV:GhCLA1 and TRV:GhABP19 vectors. The plasmids of TRV1, TRV:GhCLA1, and TRV:GhABP19 were transformed into A. tumefaciens strain GV3101 by heat shock. Then, A. tumefaciens containing TRV1 and A. tumefaciens containing TRV:GhABP19 or TRV:00 were mixed in equal amounts and injected into the cotyledons of 2-weeks-old Zhongzhimian 2 cotton cultivar seedlings to generate the GhABP19-silenced (TRV:GhABP19) cotton and control (TRV:00) cotton plants. TRV:GhCLA1 plants were used as positive controls as described previously (Gao et al., 2011) and showed clear signs of albinism in leaves after VIGS for 2 weeks (Supplementary Figure S2). TRV:GhABP19 and TRV:00 would be challenged with V. dahliae and F. oxysporum by syringe inoculation according to Liu N. et al. (2016). The assays were repeated three times (n ≥ 20), and similar results were obtained.
Measurement of H2O2 Content and SOD Activity
All Arabidopsis and Zhongzhimian 2 cotton cultivar were inoculated with two drops (5 μL) of V. dahliae and F. oxysporum conidial suspension per leaf (both of 107-conidia/mL; four leaves per plant in Arabidopsis and on the true leaves of cotton), respectively. Infected leaves were collected at 48 h post inoculation (hpi) and as samples for H2O2 content and SOD activity measurement. FOX reagent was performed to measurement the content of H2O2 in samples under normal or inoculation conditions (Cheeseman, 2006). Samples were ground with liquid nitrogen and H2O2 was extracted by adding HCl (400 μL, 25 mM) to samples, centrifuged. 100 μL supernatant was added to 900 μL FOX reagent [ammonium iron (II) sulfate (250 μM), sorbitol (100 μM), xylenol orange (100 μM), H2SO4 (25 mM), 1% ethanol] and incubated for 30 min at 30°C, A560 was measured. The total SOD activity of purified protein and endogenous SOD activity of samples were measured using a SOD assay kit (Beyotime Institute of Biotechnology, Jiangsu, China) with WST-8 method. Commercial bovine erythrocyte Cu/Zn-SOD was used as positive control (Acmec Biochemical, Shanghai, China). The assays were repeated three times (n ≥ 20), and similar results were obtained.
Expression Level of Defense-Related Genes in Arabidopsis and Cotton Plants
Total RNA was extracted from samples obtained from non-inoculated leaves with TRNzol RNA kit [TIANGEN BIOTECH (Beijing) CO., LTD.]. The procedures of synthesizing the first-strand cDNA and qRT-PCR assays were preformed just as mentioned before. The Arabidopsis housekeeping gene elongation factor 1α (EF1α) and GhUBQ7 were employed as internal standards. The relative expression was determined by the 2-ΔΔCT method and data were analyzed in Origin 8. qRT-PCR assays were carried out in three independent biological samples per treatment and three technical replicates per samples. Primers used for this analysis are shown in Supplementary Table S2.
Homology Modeling
Initial homology model of GhABP19 was generated using SWISS-MODEL workspace5 (Bordoli et al., 2009). The crystal structure of Hordeum vulgare germin (2ET7) was used as template to predict the theoretical model. All three-dimensional models were analyzed and visualized using EzMol, Version 1.206 (Reynolds et al., 2018).
Cis-Elements Analysis for the Promoter
1500 bp sequence upstream of GhABP19 was downloaded from NCBI for cis-elements analysis. The sequence was scanned by PLACE7 which is a database of nucleotide sequence motifs found in plant cis-acting regulatory DNA elements (Higo et al., 1999).
Statistical Analysis
All experiments and measurements were performed with three replicates per treatment. Analysis of variance (ANOVE) was carried out using statistical software IBM SPSS statistics 20. Data are presented as mean ± standard error. Significant differences were determined at the 5 and 1% level of significance and asterisks are used to indicate p-values: ∗p < 0.05, ∗∗p < 0.01.
Results
Characterization and Sequence Analysis of GhABP19
GhABP19 cDNA was obtained from cotton by using colony in situ hybridization, (GenBank accession number: MH430583). The full length cDNA of GhABP19 is 983 bp, including 15 bp 5′ and 233 bp 3′ untranslated regions. The 735 bp predicted open reading frame encoded a protein of 244 amino acids without a signal peptide (Figure 1A). Multiple alignments of the GhABP19 sequence with other reported true germin/GLP genes highlighted the numbers of conserved motifs and structural similarities that are common to the plant GLP subfamily. The predicted protein contains a KGD motif and three highly conserved germin/GLP oligopeptides, named boxes A, B and C (Figure 1B; Bernier and Berna, 2001).
FIGURE 1.
Sequence analysis of GhABP19 and multiple sequence alignment of germins and germin-like proteins. (A) Schematic representation of GhABP19 primary transcript. (B) Typical motifs (boxes A, B, and C) are boxed. X indicates any hydrophobic amino acid. GenBank accession numbers for the individual sequences used in this analysis are listed in Supplementary Table S1.
The putative phosphorylation sites in GhABP19 were predicted using bioinformatic analysis. There are twelve sites at serine residues (positions 18, 26, 37, 50, 76, 89, 98, 122, 168, 193, 200, and 201) and six at threonine residues (positions 80, 82, 191, 220, 226, and 227). Serine at position 200 and threonine at position 227 have the likelihood of phosphorylation since their prediction scores were higher than those of the other potential phosphorylation sites (Blom et al., 2004). The prediction for N-acetylation was negative as no alanine, glycine, serine, or threonine was present at positions 1–3 in the amino acid sequence of GhABP19. The theoretical pI of the protein was 6.88 with a molecular mass of 25,922.88 Da. The instability index was 26.49, which suggested that the protein was stable, whereas the aliphatic index was 92.75 (Ikai, 1980).
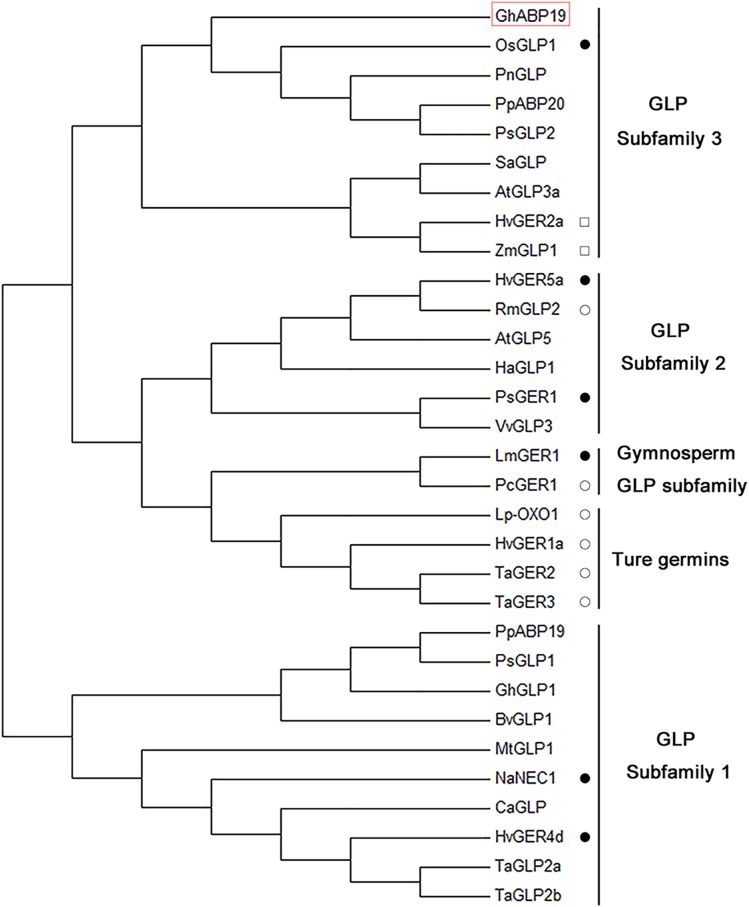
A phylogenetic tree comprising 31 germin/GLP sequences from 19 species was generated (Figure 2 and Supplementary Table S1). The dendrogram analysis revealed that germins/GLPs could be divided into five subfamilies, namely the true germin subfamily, GLP subfamily 1, GLP subfamily 2, GLP subfamily 3, and gymnosperm GLP subfamily (Carter and Thornburg, 2000). GhABP19 is a member of GLP subfamily 3, which is a very heterogeneous group. This group includes low-affinity auxin-binding proteins, such as PpABP20 form peach (Ohmiya et al., 1998) and PsGLP2 form Prunus salicina(El-Sharkawy et al., 2010); as well as GLPs known to express in a circadian pattern in Pharbitis nil (Ono et al., 1996), Sinapis alba (Heintzen et al., 1994), A. thaliana (Staiger et al., 1999), H. vulgare (Vallelian-Bindschedler et al., 1998), and Zea mays (Fan et al., 2005); and a disease resistance related protein OsGLP1 (Banerjee and Maiti, 2010).
FIGURE 2.
Phylogenetic analysis of GhABP19 together with other plant germins and germin-like proteins. The analysis involved 31 proteins; amino acid sequence of GhABP19 is in red box. Those proteins that have been analyzed for enzymatic activities are marked. Closed circles indicate the protein has superoxide dismutase activity. Open circles indicate that the protein has oxalate oxidase activity. Squares indicate that the protein has ADP glucose pyrophosphatase/phosphodiesterase activity. Sequences were aligned using MEGA 7.0 and the evolutionary history was inferred using the neighbor-joining method.
Expression Profiling of GhABP19 in Cotton Cultivars
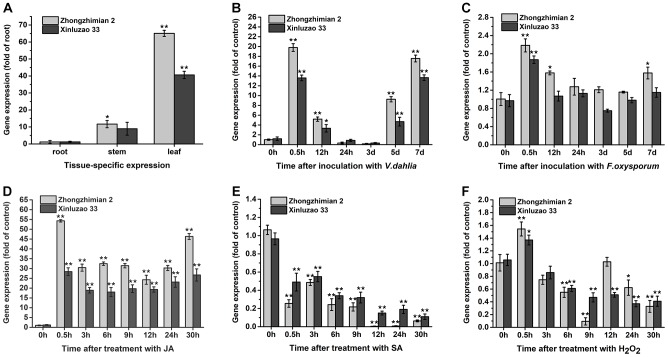
We used qRT-PCR analysis to determine the GhABP19 tissue-specific expression pattern in the resistant Zhongzhimian 2 and the susceptible Xinluzao 33 cotton cultivars, respectively. Root, stem, and leaf tissues were collected from both cotton cultivars under normal growth conditions, respectively. The results in Figure 3A indicated that GhABP19 in Zhongzhimian 2 was preferentially expressed in leaf and stem tissue, while GhABP19 only with high level of expression observed in leaf tissue in Xinluzao 33. Furthermore, these two cultivars were used to evaluate whether GhABP19 expression was regulated in response to infection with V. dahliae or F. oxysporum. As shown in Figure 3B,C, GhABP19 expression in Zhongzhimian 2 was significantly increased after inoculation with either V. dahliae and F. oxysporum for 0.5 h, 12 h, 5 days, 7 days or 0.5 h, 12 h, 7 days, respectively. The GhABP19 transcription abundance in Xinluzao 33 also can be induced by pathogen infection, but its lower than that in Zhongzhimian 2. Next, JA and SA were applied to analyze whether GhABP19 expression was related to phytohormone signaling. In both cotton cultivars, GhABP19 transcription was immediately up-regulated following JA treatment; it was maintained at a high level until 30 h post treatment (Figure 3D). In contrast, GhABP19 showed significantly reduced by SA stimulation (Figure 3E). In the presence of H2O2, GhABP19 level was slightly increased at 0.5 h in both cotton cultivars and with a minimum level observed at 9 h in Zhongzhimian 2 and 24 h in Xinluzao 33, respectively (Figure 3F).
FIGURE 3.
The expression patterns analysis of GhABP19 gene under different conditions in the resistant Zhongzhimian 2 and the susceptible Xinluzao 33 cotton cultivars. (A) Tissue-specific expression of GhABP19 gene. (B,C) GhABP19 expression 0, 0.5, 12 and 24 h, 3, 5, and 7 days after inoculation with Verticillium dahliae (B) and Fusarium oxysporum (C), control plants were treated with water. (D–F) GhABP19 expression 0, 0.5, 3, 6, 12, 24, and 30 h after treatment with JA (D), SA (E), and H2O2 (F), control plants were treated with Hoagland medium. GhABP19 expression was quantified by qRT-PCR and compared to controls. Data were collected from three independent biological samples per treatment and three technical replicates per samples. Error bars represent standard error. Asterisks indicate a significant difference compared with control (∗P < 0.05, ∗∗P < 0.01, Student’s t-test).
Antifungal Activity of Purified Recombinant GhABP19 Protein
We conducted a more detailed functional analysis of GhABP19 and produced the recombinant protein in the E. coli system. A protein with the expected molecular weight of the GhABP19 protein (25.9 kDa) was seen by SDS-PAGE after 2–5 h induction with 0.1 mM IPTG (Figure 4A). After the recombinant protein was purified using Ni columns, the elution fractions migrated as doublets on SDS gels (Figure 4B), the difference between the isoforms can be explained by the nature of germin glycan moieties (Jaikaran et al., 1990; Lane, 1994).
FIGURE 4.
Purification of recombination GhABP19 protein. (A) SDS-PAGE gel electrophoresis for prokaryotic expression of GhABP19. Expressed proteins are indicated in arrows. Lane M, protein molecular weight markers. Lanes 1, 2, 3, and 4, crude extract without IPTG induction, and cultured at 37°C with oscillation for 2, 3, 4, and 5 h, respectively; lanes 5, 6, 7, 8, crude extract with 0.1 mM IPTG induction, and cultured at 37°C with oscillation for 2, 3, 4, and 5 h, respectively. (B) SDS-PAGE of purified recombination GhABP19. Lane M, protein molecular weight markers. Lane 1–5, purifier protein with different elution times.
To investigate the antifungal activity of recombinant GhABP19 in vitro, we used a hyphal extension inhibition assay to measure the inhibitor activity of GhABP19 in response to pathogens such as V. dahliae, F. oxysporum, R. solani, B. cinerea, and V. mali. Results indicated that GhABP19 inhibited F. oxysporum and V. dahliae growth with IC50 246.58 and 298.19 μg/mL, respectively (Table 1).
Table 1.
GhABP19 IC50 against fungal pathogens.
| Pathogen | IC50 (μg/mL) |
|---|---|
| Valsa mali | 65.78 |
| Rhizoctonia solani | 75.42 |
| Botrytis cinerea | 133.59 |
| Fusarium oxysporum | 246.58 |
| Verticillium dahliae | 298.19 |
Five concentrations (35, 70, 140, 280, and 560 μg/mL) of purified GhABP19 were used for determination the IC50 of antifungal activity. Buffer was used as a control.
SOD Structural Domain in GhABP19 and Its SOD Activity
To understand the antifungal mechanism of GhABP19, we generated a three-dimensional model of this protein via homology modeling to investigate the presence of a domain to perform the function of fungal resistance. Barley germin (PDB number: 2ET7) was used as a template (Figure 5A) and showed 40.22% similarity to GhABP19. The GhABP19 model predicted active sites composed of three histidine (His137, His139, and His193) and one glutamate (Glu144) residues responsible for metal ion binding and SOD activity (Figure 5B). The active sites, analogous to barley germin, are protected within the jellyroll β-barrel structures, which are characteristic of the cupin superfamily.
FIGURE 5.
Structure predictions of GhABP19 and its SOD activity. (A,B) Modelings of Hordeum vulgare germin protein (A) and GhABP19 protein (B). The H. vulgare germin amino acids–His88, His90, His137, and Glu97 and GhABP19 amino acids–His137, His139, His183, and Glu144 responsible for SOD domain are highlighted. (C) SOD activity of purified GhABP19 protein. 4.4, 8.1, 12.4, and 16.0 μg of purified GhABP19 protein was used in this assay. Commercial bovine erythrocyte Cu/Zn-SOD was used as positive control. Error bars represent standard error and shown for three replicates.
Furthermore, we experimentally identified the SOD activity of the recombinant GhABP19 protein, which was predicated by structural features. SOD generates H2O2 and O2 by converting the superoxide radical anion (O2-). Thus, the most crucial procedure for determining the enzymatic ability of SOD is based on superoxide anion–dependent inhibiting reactions. The SOD activity was evaluated using a SOD assay kit (Beyotime Institute of Biotechnology, Jiangsu, China), in which WST-8 was used to produce a water-soluble formazan dye upon reduction with the superoxide anion generated by xanthine oxidase. SOD can inhibit this reaction by removing superoxide anion. The SOD activity could be measured using a colorimetric method. We used 4.4, 8.1, 12.4, and 16.0 μg GhABP19 protein to perform this assay (Figure 5C). Commercial bovine erythrocyte Cu/Zn-SOD was used as positive control (Acmec Biochemical, Shanghai, China).
Subcellular Localization
The subcellular localization of GhABP19 was experimentally determined using GhABP19-GFP fusion protein expressed in the Arabidopsis seedling root cells, the GFP fluorescence was observed in extracellular space by Confocal Laser Scanning Microscopy (Figure 6). The result from Figure 6B showed that GhABP19 was located in the cell wall or in the plasma membrane. The seedlings were treated with 0.8 M mannitol for 10 min to differentiate between the plasma membrane and cell wall location. After plasmolysis, the GFP fluorescence in Figure 6C revealed that GhABP19 was localized in the plasma membrane, which was consistent with the predictions conducted by TMHMM Server v 2.08, WoLF PSORT9 and DeepLoc-1.010.
FIGURE 6.

Subcellular localization of GhABP19-GFP fusion protein in Arabidopsis root cells. (A) Control (35S-GFP) under empty vector. (B) GhABP19-GFP fusion protein. (C) Plasmolyzed GhABP19-GFP fusion protein. Plasmolysis was induced with 0.8 M mannitol for 10 min. Scale bar represents 50 μm.
GhABP19 Overexpression in Arabidopsis in Response to V. dahliae and F. oxysporum in Soil
The presence and expression of GhABP19 in hygromycin-resistant Arabidopsis lines were identified by genomic PCR analysis (data not shown). Homozygous transgenic (T3 generation) lines of L1, L3, and L4 were selected for subsequent experiments based on qRT-PCR analysis, the transcript levels were normalized relative to the line with the lowest transgenic expression (L10; Supplementary Figure S1B).
To verify the antifungal activity of the GhABP19 gene in plants, GhABP19-transgenic Arabidopsis plants were infected with V. dahliae and F. oxysporum in soil, respectively. For the fungus infection assay, 4-week-old transgenic and control Arabidopsis plants were infected by dipping the roots in fungal culture and co-cultivated in a growth chamber, in which non-infected plants served as a control. Disease incidence and severity were estimated using the PDI. For V. dahliae infection, clear differences were noted between wild-type and transgenic plants at 30 days post inoculation (dpi): wilting, yellowish color, and necrosis appeared on most leaf surfaces of control plants, whereas transgenic plants grew regularly, despite slight disease symptoms being visible on some leaves (Figure 7A). Plants of transgenic L1, L3, and L4 lines showed lower PDI (73.8 ± 1.1%, 75.5 ± 2.21%, and 77.8 ± 1.29%, respectively) compared with those of the control plants (85.9 ± 3.18%) at 30 dpi (Figure 7B). With regard to F. oxysporum infection, more than 70.4 ± 1.2% of the leaves of control plants were dead at 49 dpi. Compared with this result, symptoms detected on the older leaves and branches of transgenic plants were considerably milder (Figure 7A,C). These results are consistent with the antifungal activity of the GhABP19 protein and showed that the expression of GhABP19 in Arabidopsis reduces the susceptibility of plants to pathogenic infection.
FIGURE 7.
Resistance and analysis of H2O2 content, endogenous SOD activity in transgenic Arabidopsis plants. (A) Non-infected control (empty vector) and GhABP19-transgenic Arabidopsis plants, and Arabidopsis plants infected with V. dahliae and F. oxysporum in soil 30 and 49 dpi, respectively. (B,C) Plant disease indexes of control and transgenic Arabidopsis plants at the indicated days after inoculation with V. dahliae (B) and F. oxysporum (C). Error bars represent the standard error of three biological replicates (n ≥ 30). (D) H2O2 content and (E) endogenous SOD activity in the leaves of control and GhABP19-transgenic Arabidopsis lines before and after inoculation with V. dahliae and F. oxysporum at 48 hpi. Error bars represent the standard error of three biological replicates (n ≥ 20). Asterisks indicate a significant difference compared with control (∗P < 0.05, ∗∗P < 0.01, Student’s t-test). FW, fresh weight; Ctrl, Control; Line 1, Line 3, Line 4, transgenic Arabidopsis lines.
Resistance of Transgenic Arabidopsis Seedlings to F. oxysporum
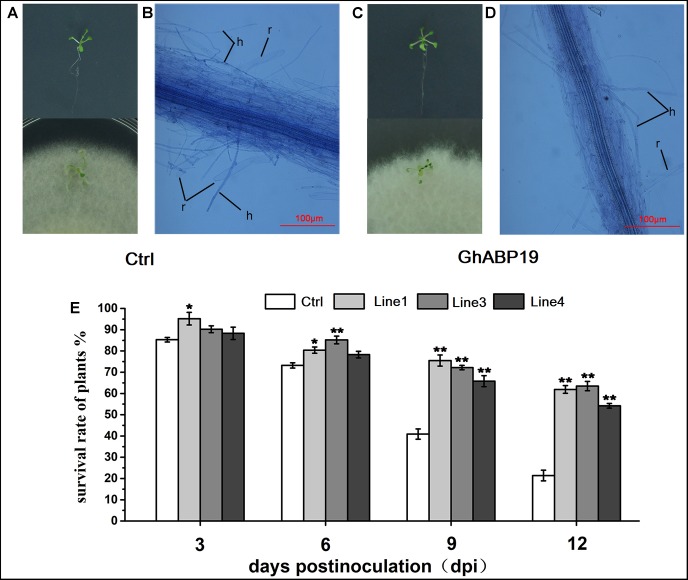
To substantiate the data obtained from infection experiments in soil, seedlings of control and GhABP19-transgenic Arabidopsis line with the highest GhABP19 expression level (L1) were infected with F. oxysporum on agar plates to observe the penetration and development conditions of hyphae. Firstly, control and transgenic plants were transferred to agar plates inoculated with F. oxysporum mycelium and co-cultured in a growth chamber. Twelve days after infection, control Arabidopsis plants were severely infected with fungus (Figure 8A,B). They showed strong disease symptoms and 78.6 ± 2.5% died after infection (Figure 8E). However, transgenic plants only showed mild wilt (Figure 8C,D), and more than 54.2 ± 1.1% still grew normally (Figure 8E).
FIGURE 8.
Infection assays with F. oxysporum on Arabidopsis seedlings and light microscopic observation of the F. oxysporum infection process on Arabidopsis seedlings roots. (A) Control (empty vector) Arabidopsis plants grow on MS plate before infection and 12 dpi with F. oxysporum. (B) Attachment and directed-growth of F. oxysporum hyphae over the root of control Arabidopsis plant. (C) GhABP19 transgenic Arabidopsis grow on MS plate before infection and 12 dpi with F. oxysporum. (D) F. oxysporum growing on the root surface of GhABP19 transgenic Arabidopsis plant without firm attachment. (E) Survival rate of control and transgenic Arabidopsis seedlings infected with F. oxysporum. Error bars represent the standard error of three biological replicates (n ≥ 20). Asterisks indicate a significant difference compared with control (∗P < 0.05, ∗∗P < 0.01, Student’s t-test). r, root; h, hyphae; Ctrl, Control; Line 1, Line 3, Line 4, transgenic Arabidopsis lines.
For microscopic observation of the F. oxysporum infection process, the infected roots were stained with trypan blue solution at 12 dpi. The roots were flushed with running tap water to remove most of the non-attached mycelium. Compared with the control roots, the hyphae on the root surface and mycelium inside the roots of transgenic Arabidopsis were remarkably reduced (Figure 8B,D). This indicated that both the penetration and development of the fungus in transgenic Arabidopsis roots were strongly inhibited. In conclusion, the microscopic observations supported the findings of the infection experiments in soil in that GhABP19 inhibited fungal infections in transgenic Arabidopsis plants.
GhABP19 Silencing and Cotton Resistance to V. dahliae and F. oxysporum
Virus-induced gene silencing is a powerful tool for determining the various functions of genes (Lou and Baldwin, 2006; Gao et al., 2011; Mejía-Teniente et al., 2015). To clarify the role of GhABP19 in the defense response of cotton against V. dahliae and F. oxysporum, we used Agrobacterium-mediated VIGS to generate GhABP19-silenced Zhongzhimian 2 cotton cultivar (TRV:GhABP19) and control plants (TRV:00). Further, the cotton gene GhCLA1, that is involved in chloroplast development and which needed to be silenced, would be a visual marker to monitor VIGS efficiency (Gao et al., 2011). The silencing of GhABP19 and GhCLA1 was confirmed by semi-quantitative RT-PCR and qRT-PCR analysis after 2 weeks of VIGS (Figure 9C and Supplementary Figure S2). The results showed that GhABP19 was successfully knocked down.
FIGURE 9.
Susceptibility of GhABP19-silenced Zhongzhimian 2 cotton cultivar to V. dahliae and F. oxysporum. (A) Disease symptoms of control (TRV:00) and GhABP19-silenced (TRV:GhABP19) cotton plants infected by V. dahliae and F. oxysporum 18 dpi. (B) Representative leaves and vascular browning in stem of the control and GhABP19-silenced cotton plants infected by V. dahliae and F. oxysporum 18 dpi. (C) Mean expression levels of GhABP19 gene analyzed by qPR-PCR. Total RNAs were extracted from the leaves of cotton plants after 2 weeks of VIGS, and the expression level of GhABP19 in VIGS plants was compared with that of the control plants (TRV:00). Error bars represent the standard error of three biological replicates (n ≥ 20). Asterisks indicate a significant difference compared with control (∗P < 0.05, Student’s t-test). (D) Disease index of control and GhABP19-silenced cotton plants at the indicated days after inoculation with V. dahliae and F. oxysporum, respectively. Error bars represent the standard error of three biological replicates (n ≥ 30). (E) H2O2 content and (F) endogenous SOD activity in the leaves of control and GhABP19-silenced cotton plants before and after inoculation with V. dahliae and F. oxysporum, respectively. Error bars represent the standard error of three biological replicates (n ≥ 20). Asterisks indicate a significant difference compared with non-infection plants (∗P < 0.05, ∗∗P < 0.01, Student’s t-test). FW, fresh weight.
To investigate the role of GhABP19 in cotton, control and GhABP19-silenced Zhongzhimian 2 were challenged with spore suspensions of V. dahliae and F. oxysporum by syringe inoculation. As results, yellow wilted leaves appeared 6 days after inoculation in GhABP19-silenced cotton. After 18 days, there were severe necrosis symptoms in the leaves of GhABP19-silenced plants (Figure 9A,B). The vertical vascular tissue in GhABP19-silenced plant stems showed more serious discoloration than that of control plants (Figure 9B). The plant disease index of control plants was much higher than that of GhABP19-silenced plants after inoculation with V. dahliae and F. oxysporum, respectively (Figure 9D). The disease symptoms of cotton plants suggested that silencing GhABP19 increased plants’ susceptibility to V. dahliae and F. oxysporum infection.
H2O2 Accumulation and Endogenous SOD Activity in Transgenic Arabidopsis Lines and GhABP19-Silenced Cotton Plants
Previous studies have shown that several GLPs exhibit SOD activity, which leads to H2O2 production and is often correlated with plant defense responses (Sultana et al., 2016; Zhang et al., 2017, 2018). In our study, the recombinant GhABP19 protein exhibited SOD activity in vitro. To confirm whether the enhanced disease resistance in GhABP19-transgenic Arabidopsis plants is related to H2O2 accumulation resulting from endogenous SOD activity of GhABP19, we analyzed the H2O2 content and endogenous SOD activity in transgenic Arabidopsis plants. After inoculation with V. dahliae and F. oxysporum, respectively, all the GhABP19 transgenic lines showed significantly higher H2O2 levels compared to those in control plants at 48 hpi (Figure 7D). Moreover, the endogenous SOD activity was determined in the same plants used for H2O2 quantification. As shown in Figure 7E, endogenous SOD activity in transgenic Arabidopsis plants was elevated before and after pathogen inoculation, respectively.
In addition, the H2O2 contents and SOD activity of TRV:00 and TRV:GhABP19 plants were measured after inoculation with fungi. The levels of H2O2 and SOD activity in non-infected TRV:00 and TRV:GhABP19 exhibited little difference. However, endogenous H2O2 contents and SOD activity increased significantly in both TRV:00 and TRV:GhABP19 after pathogen inoculation (Figure 9E,F), suggesting that the silence of GhABP19 is responsible for reducing H2O2 production and SOD activity.
The Expression of Defense-Related Genes Involved in JA Signaling Pathway in Transgenic Arabidopsis Lines and GhABP19-Silenced Cotton Plants
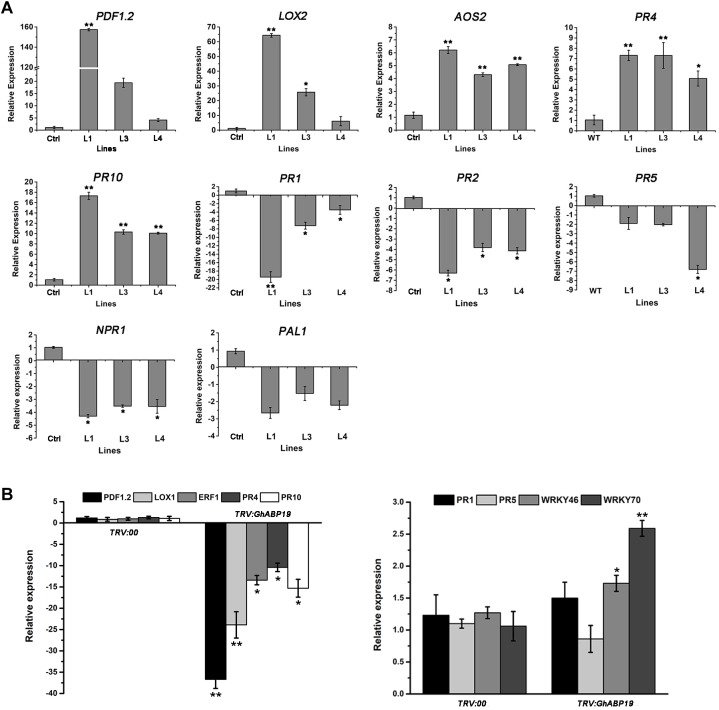
Based on the observation that GhABP19 expression was induced by JA but depressed by SA treatments, we examined whether the resistance conferred by GhABP19 observed in this study was correlated with the induction of JA and/or SA endogenous defense signaling pathways. The expression patterns of some defense-related marker genes involved in the JA (PDF1.2, LOX2, AOS2, PR4, and PR10) and SA pathway (PR-1, PR-2, PR-5, NPR1, and PAL1) were detected in non-inoculated GhABP19-transgenic Arabidopsis lines and control plants by qRT-PCR. Even without pathogen infection, JA pathway–related AOS2, PR4, and PR10 were strongly induced in all GhABP19-transgenic lines compared with the control plants (Figure 10A). In contrast, the expression levels of PR-1, PR-2, and NPR1, which are associated with the SA pathway, were significantly downregulated in GhABP19-transgenic Arabidopsis lines, and there was no difference in the expression of PR-5 and PAL1 genes (Figure 10A).
FIGURE 10.
qRT-PCR analyses of JA- and SA-related genes. (A) The relative expression of genes in non-inoculated control and GhABP19-transgenic Arabidopsis lines. AtEF1α was used as an internal standard. (B) The relative expression of genes in non-inoculated TRV:00 and TRV:GhABP19 cotton plants. GhUBQ7 was used as an internal standard. The fold down-regulation was presented as calculated by the formula –1/normalized gene expression value. Data were collected from three independent biological samples with three technical replicates. Error bars represent the standard error. Asterisks indicate a significant difference compared with control (∗P < 0.05, ∗∗P < 0.01, Student’s t-test). Ctrl, control; L1, L3, L4, transgenic Arabidopsis lines.
We also analyzed the expression levels of several well-characterized JA- and SA-related defense genes in non-inoculated GhABP19-silenced cotton plants. The expression levels of PDF1.2, LOX1, ERF1, PR4, and PR10, which are involved in JA-related defense responses, were significantly down-regulated by GhABP19 suppression in cotton (Figure 10B). Silencing of GhABP19 did not alter the transcripts of SA-related genes PR1 and PR5, however, the expression of WRKY46 and WRKY70 in SA signal pathway were increased in the GhABP19-silenced plants (Figure 10B).
Analysis of cis-Elements in GhABP19 Promoter
To analyze the pathogen resistance–related cis-elements in GhABP19 promoter, the 1500 bp promoter sequence from the transcription start site of GhABP19 was predicted by PLACE. The putative results revealed that several regions might be important for the pathogen resistance conferred by the gene. Three types of pathogen resistance-related elements were included in the GhABP19 promoter (Table 2 and Supplementary Figure S3). The GT-1-box in the promoter of the Glycine max SCaM-4gene plays a role in pathogen- and salt-induced SCaM-4 gene expression (Park et al., 2004). Two sequences similar to the GT-1-box (GAAAAA) existed at positions -990 and -561 in GhABP19, respectively. Furthermore, WRKY proteins bind in vitro to functionally define the elicitor-response elements of the W-box elements [(T)TGAC(C)] present in PR1 promoters (Eulgem et al., 1999). We found four W-box elements in the studied promoter, including two TGAG-containing W-box elements (TGAC) at positions -1410 and -165 and two elicitor responsive elements [ELER, TTGAC(C)] at positions -757 and -683, respectively. In addition, the binding site of the potato silencing element-binding factor (CTGTCAC) was found in the promoter of the pathogen resistance–related gene PR-10a(Boyle and Brisson, 2001), and a related element was found at position -29 in GhABP19. These three kinds of cis-elements might be involved in the fungal resistance of GhABP19. However, further studies are needed to investigate their specific functions.
Table 2.
Pathogenesis-related elements in the promoter of GhABP19.
| Gene | Sequence | References |
|---|---|---|
| (a) GT-1 BOX | ||
| Gm SCaM-4 | GAAAAA | Park et al., 2004 |
| ABP19 | GAAAAA (990/561) | This paper |
| (b) W-box | ||
| TGAG-containing W box element | ||
| Pc WRKY1 | TGAC | Eulgem et al., 1999 |
| ABP19 | TGAC (1410/165) | This paper |
| ELER (elicitor responsive element) | ||
| Pc PR1 | TTGACC | Eulgem et al., 1999 |
| ABP19 | TTGACC (757), TTGAC (683) | This paper |
| (c) SEBF (silencing element binding factor) | ||
| St PR-10a | CTGTCAC | Boyle and Brisson, 2001 |
| ABP19 | TTGTCAC (29) | This paper |
Discussion
Germin-like proteins (GLPs) are plant glycoproteins and a crucial component of plant basal host resistance (Zimmermann, 2006). In the present study, we characterized GhABP19, a novel GLP gene isolated from cotton and provided evidence for its role in the regulation of resistance to verticillium and fusarium wilt in plants.
Plant GLPs have been classified into five subfamilies (Carter and Thornburg, 2000), GhABP19 belongs to the GLP subfamily 3 and shares a common characteristic with other members of this group: they highly abundant in young leaves, less abundant in stems, and absent in roots (Figure 2, 3A; Heintzen et al., 1994; Ono et al., 1996; Vallelian-Bindschedler et al., 1998; Fan et al., 2005).
The general structure of GhABP19 is consistent with the typical organization of germins and GLPs (Figure 1B). In most of the GLP family members, box-A consists of a consensus sequence (QDFCVAD) (Bernier and Berna, 2001). However, GhABP19 is predicted to possess a sequence variation, as glutamine (Q) and alanine (A) are replaced by alanine (A) and glycine (G), respectively. In addition, a cysteine (C) residue at position 62 is included in GhABP19 box-A, which is followed by a second cysteine (C) at position 77, and could form an internal disulfide bridge of the extracellular domain (Woo et al., 2000). Box-B(G–P-H-HPGASEXXXXX-G), corresponding to amino acid residues from 132 to 151, is conserved at the inner position of the full-length sequence. Box-C is GXXHFQ-N-G, where X corresponds to any hydrophobic amino acid residue. There are two histidine (H) and one glutamate (E) residues in box-B, and box-C contains the third histidine residue, which in germins is involved in heavy metal ion-binding (Gane et al., 1998) and considered to be the ligand-binding conserved sequence in the auxin-binding protein/germin class (Dunwell and Gane, 1998; Woo et al., 1998). The RGD-like tripeptide motif sequence, detected in over 50% of GLPs characteristically involved in protein–protein interactions, was also present (Figure 1B; Labour et al., 1999). In animal cells, these tripeptide domains are found in cell adhesion proteins from the extracellular matrix (such as vitronectin and fibronectin) that interact with transmembrane proteins called integrins (Labour et al., 1999).
The results of bioinformatic analysis and subcellular localization experiments showed that GhABP19, is a transmembrane protein distributed at plasma membrane or cell wall (Figure 6B). After plasmolysis, GhABP19 was confirmed to locate in the plasma membrane (Figure 6C), which was consistent with the localization of several GLPs in Arachis hypogaea (Wang et al., 2013).
Previous studies have shown that GLP genes might be essential for plant general resistance to biotic stress; the expression of GLPs is differentially regulated in response to pathogen infection in different plant species (Breen and Bellgard, 2010). For instance, GLPs in B. vulgaris, Brassica napus, A. hypogaea, and L. regale show a broad spectrum of defensive activities in host–pathogen interactions (Knecht et al., 2010; Rietz et al., 2012; Wang et al., 2013; Zhang et al., 2017). In this study, we used the resistant Zhongzhimian 2 and the susceptible Xinluzao 33 cotton cultivars to investigate a possible role of GhABP19 gene in response to V. dahliae and F. oxysporum infection. The result reported in the literature indicate that the susceptible cultivar exhibited a little more accumulated increased fungal biomass when compared with the resistant cultivar in both V. dahliae and F. oxysporum; this may be partially reflected in the difference between resistant and susceptible varieties (Liu et al., 2017a). Our data showed that the GhABP19 gene in two non-treated cotton cultivars both showed high transcript abundance in the leaf tissue, indicating conserved gene regulation under normal growth conditions (Figure 3A). After inoculation with V. dahliae and F. oxysporum, there was first an increase in GhABP19 expression, followed by its decrease in both cotton cultivars, and the expression levels of GhABP19 in Zhongzhimian 2 were a bit higher than that in Xinluzao 33 (Figure 3B,C), suggesting a possible role in plant basal resistance. Notably, an obvious difference existed between the expression levels of GhABP19 induced by V. dahliae and F. oxysporum, respectively. Take Zhongzhimian 2 for example, the expression of GhABP19 was up-regulated by about 20-fold after inoculation with V. dahliae at 0.5 hpi, but it was only up-regulated by 2.2-fold when treated with F. oxysporum at the same hpi. Based on this result, we predicted that GhABP19 is considerably more instrumental in enhancing the resistance of cotton to V. dahliae infection than it is to F. oxysporum infection.
Arabidopsis transgenic technology and silencing the endogenous genes through VIGS are two convenient methods for gene function characterization, and these approaches were employed to determine the role of GhABP19 in Arabidopsis and cotton plants, respectively. The GhABP19-transgenic Arabidopsis lines were infected with V. dahliae and F. oxysporum to assess their resistance responses, respectively (Figure 7). The results revealed that overexpression of GhABP19 in Arabidopsis plants enhanced their disease resistance, reducing chlorosis and death of transgenic plants. Furthermore, microscopic observation of Arabidopsis roots infected with F. oxysporum provided evidence for the accumulation of GhABP19, which could prevent the penetration and development of the fungus (Figure 8). In GhABP19-silenced cotton plants, GhABP19 silencing reduced V. dahliae and F. oxysporum resistance of cotton seedlings, as determined by pathogen inoculation assays (Figure 9). Furthermore, the necrosis of leaves and the discoloration of the vascular network in GhABP19-silenced cotton plants caused by V. dahliae are more serious than that caused by F. oxysporum (Figure 9).
Reactive oxygen species, especially H2O2, are generated in pathogen-infected plants. H2O2 plays various roles in host–pathogen interactions, and it is suggested as an antimicrobial agent in plant defense responses (Walters, 2003). It is further involved in different signaling pathways associated with defense mechanisms, such as triggering of the hypersensitivity response, accumulation of phytoalexins, and activation of many other defense-response genes (Shetty et al., 2008). However, overaccumulation of reactive oxygen species in plant cells damages biomolecular components, such as membrane lipids, nucleic acids, chloroplast pigments, and proteins (Verma and Dubey, 2003). To overcome oxidative damage, plants have an antioxidant defense system comprising various enzymes, for example, SOD can remove, neutralize, and scavenge reactive oxygen species by converting O2- to H2O2 and O2 (Shah et al., 2001).
In our study, the recombinant GhABP19 protein exhibited SOD activity (Figure 5), and hyphal extension inhibition assays provided evidence of direct antifungal activity of GhABP19 on various pathogens (Table 1). This suggests that the resistance of the GhABP19 protein observed in the in vivo experiment can be attributed to the direct effect of SOD activity. Moreover, the H2O2 content and endogenous SOD activity were measured in GhABP19-transgenic Arabidopsis plants and GhABP19-silenced cotton plants after V. dahliae and F. oxysporum infection (Figure 7D,E, 9E,F). Notably, H2O2 content and SOD activity were elevated in GhABP19-transgenic Arabidopsis plants compared to control plants; and as expected, silencing of GhABP19 decreased the accumulation of H2O2 and the activity of endogenous SOD enzyme. One possible explanation for the role of GhABP19 in plant fungal resistance could therefore be the accumulation of H2O2 produced by SOD activity.
Although the increase in H2O2 might be a GhABP19-mediated defense response to fungal infection, it remains unclear whether this increase leads to the activation of any signaling pathways. JA and SA are important phytohormones in regulating plant disease resistance (Thatcher et al., 2005). Given the GhABP19 expression was induced after treatment with exogenous JA but suppressed by SA treatment (Figure 3D,E), we hypothesized that GhABP19 likely involved in phytohormone signaling regulation. Here, the expression of some genes involved in the SA and JA pathways was analyzed. In Arabidopsis plants, the overexpression of GhABP19 activated the transcript levels for AOS2, PR4, and PR10, which depend on the JA signaling pathway but decreased the expression levels of selected genes associated with the SA pathway, such as PR-1, PR-2, and NPR1 (Figure 10A). The activation of JA-related genes in the transgenic Arabidopsis lines even in the absence of pathogens better protected the plant. Moreover, PDF1.2, LOX1, ERF1, PR4, and PR10 in JA signal pathway were suppressed in non-inoculated GhABP19-silenced cotton plants, and minimal changes were identified in the levels of expression of genes associated with the SA-signal pathway, such as PR1 and PR5 (Figure 10B). Meanwhile, the expression levels of WRKY46 and WRKY70, which are involved in SA-related defense responses, were up-regulated by GhABP19 suppression in cotton without pathogen infection. These results revealed that several defense-related genes in JA signal pathway were suppressed by silencing GhABP19. GhABP19 is functional as a JA-signaling component in plant resistance mechanisms by specifically regulating the expression of a set of plant defense-related genes before pathogen infection.
Conclusion
The functional analysis of GhABP19, a GLP protein with SOD activity, revealed a potential role in plant disease resistance. Our findings show that ectopic overexpression of GhABP19 protected transgenic Arabidopsis against V. dahliae and F. oxysporum infection. Conversely, resistance to V. dahliae and F. oxysporum was decreased by silencing GhABP19 in cotton. The disease defensive function of GhABP19 was exerted by generating H2O2 via its SOD activity, as well as upregulating the transcription levels of several defense-related genes involved in JA pathways. These results highlight the potential application of GhABP19 in biotechnology and provide a basis for developing strategies to improve disease resistance in cotton plants. Moreover, several defense-related cis-elements are present in the promoter of GhABP19 (Supplementary Figure S3 and Table 2), but their exact functions are not yet known and need to be determined in the future.
Author Contributions
YP, YH and FL conceived and designed the study. YP conducted most of the experiments, analyzed the data, and wrote the manuscript. XL, YZ, XG, and YJ provided technical assistance to YP. YS and NL provided analysis tools. All authors reviewed and revised the manuscript and figures.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Prof. Yule Liu from Tsinghua University for the VIGS constructs.
Funding. This work was sponsored by National Key R&D Program of China “chemical fertilizers and pesticide reduction efficiency integrated technology research and development” (Grant No. 2017YFD0201900); the “Seven Crop Breeding” National Major Project (Grant No. 2016YFD0101006); the Genetically Modified Organism Breeding Major Project (Grant No. 2018ZX08005001-002); and the State Key Laboratory of Cotton Biology (Grant No. CB2017B03).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2019.00583/full#supplementary-material
References
- Banerjee J., Maiti M. K. (2010). Functional role of rice germin-like protein 1 in regulation of plant height and disease resistance. Biochem. Bioph. Res. Commun. 394 178–183. 10.1016/j.bbrc.2010.02.142 [DOI] [PubMed] [Google Scholar]
- Bernier F. O., Berna A. (2001). Germins and germin-like proteins: plant do-all proteins. But what do they do exactly? Plant Physiol. Biochem. 39 545–554. 10.1016/s0981-9428(01)01285-2 [DOI] [Google Scholar]
- Blom N., Sicheritzpontn T., Gupta R., Gammeltoft S., Brunak S. (2004). Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics 4 1633–1649. 10.1002/pmic.200300771 [DOI] [PubMed] [Google Scholar]
- Bordoli L., Kiefer F., Arnold K., Benkert P., Battey J., Schwede T. (2009). Protein structure homology modeling using SWISS-MODEL workspace. Nat. Protoc. 4 1–13. 10.1038/nprot.2008.197 [DOI] [PubMed] [Google Scholar]
- Boyle B., Brisson N. (2001). Repression of the defense gene PR-10a by the single-stranded DNA binding protein SEBF. Plant Cell 13 2525–2537. 10.1105/tpc.13.11.2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen J., Bellgard M. (2010). Germin-like proteins (GLPs) in cereal genomes: gene clustering and dynamic roles in plant defence. Funct. Integr. Genomic 10 463–476. 10.1007/s10142-010-0184-1 [DOI] [PubMed] [Google Scholar]
- Carter C., Thornburg R. W. (2000). Tobacco nectarin I. purification and characterization as a germin-like, manganese superoxide dismutase. J. Biol. Chem. 275 36726–36733. 10.1074/jbc.m006461200 [DOI] [PubMed] [Google Scholar]
- Cheeseman J. M. (2006). Hydrogen peroxide concentrations in leaves under natural conditions. J. Exp. Bot. 57:2435. 10.1093/jxb/erl004 [DOI] [PubMed] [Google Scholar]
- Cheng X., Huang X., Liu S., Tang M., Hu W., Pan S. (2014). Characterization of germin-like protein with polyphenol oxidase activity from Satsuma mandarine. Biochem. Bioph. Res. Commun. 449 313–318. 10.1016/j.bbrc.2014.05.027 [DOI] [PubMed] [Google Scholar]
- Clough S., Bent A. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16 735–743. 10.1046/j.1365-313x.1998.00343.x [DOI] [PubMed] [Google Scholar]
- Dunwell J. M., Gane P. J. (1998). Microbial relatives of seed storage proteins: conservation of motifs in a functionally diverse superfamily of enzymes. J. Mol. Evol. 46 147–154. 10.1007/pl00006289 [DOI] [PubMed] [Google Scholar]
- Dunwell J. M., Gibbings J. G., Mahmood T., Naqvi S. M. S. (2008). Germin and germin- like proteins: evolution, structure, and function. Crit. Rev. Plant Sci. 27 342–375. 10.1080/07352680802333938 [DOI] [Google Scholar]
- El-Sharkawy I., Mila I., Bouzayen M., Jayasankar S. (2010). Regulation of two germin-like protein genes during plum fruit development. J. Exp. Bot. 61 1761–1770. 10.1093/jxb/erq043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulgem T., Rushton P. J., Schmelzer E., Hahlbrock K., Somssich I. E. (1999). Early nuclear events in plant defence signaling: rapid gene activation by WRKY transcription factors. EMBO J. 18 4689–4699. 10.1093/emboj/18.17.4689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z., Gu H., Chen X., Song H., Wang Q., Liu M., et al. (2005). Cloning and expression analysis of Zmglp1, a new germin-like protein gene in maize. Biochem. Biophys. Res. Commun. 331 1257–1263. 10.1016/j.bbrc.2005.04.045 [DOI] [PubMed] [Google Scholar]
- Fradin E. F., Thomma B. P. (2006). Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Plant Mol. Biol. 7 71–86. 10.1111/j.1364-3703.2006.00323.x [DOI] [PubMed] [Google Scholar]
- Fravel D., Olivain C., Alabouvette C. (2003). Fusarium oxysporum and its biocontrol. New Phytol. 157 493–502. 10.1046/j.1469-8137.2003.00700.x [DOI] [PubMed] [Google Scholar]
- Gane P. J., Warwicker J., Dunwell J. M. (1998). Modeling based on the structure of vicilins predicts a histidine cluster in the active site of oxalate oxidase. J. Mol. Evol. 46:488. 10.1007/pl00006329 [DOI] [PubMed] [Google Scholar]
- Gao X., Wheeler T., Li Z., Kenerley C. M., He P., Shan L. (2011). Silencing GhNDR1 and GhMKK2 compromises cotton resistance to Verticillium wilt. Plant J. 66 293–305. 10.1111/j.1365-313X.2011.04491.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey D., Able A. J., Dry I. B. (2007). Induction of a grapevine germin-like protein (VvGLP3) gene is closely linked to the site of erysiphe necator infection: a possible role in defense. Mol. Plant Microbe Interact. 20 1112–1125. 10.1094/mpmi-20-9-1112 [DOI] [PubMed] [Google Scholar]
- Heintzen C., Fischer R., Melzer S., Kappeler K., Apel K., Staiger D. (1994). Circadian oscillations of a transcript encoding a germin-like protein that is associated with cell walls in young leaves of the long-day plant Sinapis alba L. Plant Physiol. 106 905–915. 10.1104/pp.106.3.905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo K., Ugawa Y., Iwamoto M., Korenaga T. (1999). Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic. Acids Res. 27 297–300. 10.1093/nar/27.1.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelbach A., Liu L., Zierold U., Altschmied L., Maucher H., Beier F., et al. (2010). Promoters of the barley germin-like GER4 gene cluster enable strong transgene expression in response to pathogen attack. Plant Cell 22 937–952. 10.1105/tpc.109.067934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikai A. (1980). Thermostability and aliphatic index of globular proteins. J. Bio. Chem. 88 1895–1898. [PubMed] [Google Scholar]
- Jaikaran A. S., Kennedy T. D., Dratewkakos E., Lane B. G. (1990). Covalently bonded and adventitious glycans in germin. J. Biol. Chem. 265 12503–12512. [PubMed] [Google Scholar]
- Kiemer L., Bendtsen J. D., Blom N. (2005). NetAcet: prediction of N-terminal acetylation sites. Bioinformatics 21:1269. 10.1093/bioinformatics/bti130 [DOI] [PubMed] [Google Scholar]
- Knecht K., Seyffarth M., Desel C., Thurau T., Sherameti I., Lou B., et al. (2010). Expression of BvGLP-1 encoding a germin-like protein from sugar beet in Arabidopsis thaliana leads to resistance against phytopathogenic fungi. Mol. Plant Microbe Interact. 23 446–457. 10.1094/MPMI-23-4-0446 [DOI] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Tamura K. (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33:1870. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labour A. M., Faik A., Mandaron P., Falconet D. (1999). RGD-dependent growth of maize calluses and immunodetection of an integrin-like protein. FEBS Lett. 442 123–128. 10.1016/s0014-5793(98)01634-2 [DOI] [PubMed] [Google Scholar]
- Lane B. G. (1994). Oxalate, germin, and the extracellular matrix of higher plants. FASEB J. 8 294–301. 10.1096/fasebj.8.3.8143935 [DOI] [PubMed] [Google Scholar]
- Leon J., Lawton M. A., Raskin I. (1995). Hydrogen peroxide stimulates salicylic acid biosynthesis in tobacco. Plant Physiol. 108 1673–1678. 10.1104/pp.108.4.1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Pei Y., Sun Y., Liu N., Wang P., Liu D., et al. (2018). A cotton cyclin-dependent kinase e confers resistance to Verticillium dahliae mediated by jasmonate-responsive pathway. Front. Plant. Sci. 9:642. 10.3389/fpls.2018.00642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Ma X., Sun Y., Hou Y. (2017a). Necrotizing Activity of Verticillium dahliae and Fusarium oxysporum f. sp.vasinfectum Endopolygalacturonases in Cotton. Plant Dis. 101 1–11. 10.1094/PDIS-05-16-0657-RE [DOI] [PubMed] [Google Scholar]
- Liu N., Zhang X., Sun Y., Wang P., Li X., Pei Y., et al. (2017b). Molecular evidence for the involvement of a polygalacturonase-inhibiting protein, GhPGIP1, in enhanced resistance to Verticillium and Fusarium wilts in cotton. Sci. Rep. 7:39840. 10.1038/srep39840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Ma X., Zhou S., Wang P., Sun Y., Li X., et al. (2016). Molecular and functional characterization of a polygalacturonase-inhibiting protein from Cynanchum komarovii that confers fungal resistance in Arabidopsis. PLoS One 11:e146959. 10.1371/journal.pone.0146959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Yang J., Yan S., Zhang S., Zhao J., Wang W., et al. (2016). The germin-like protein OsGLP2-1 enhances resistance to fungal blast and bacterial blight in rice. Plant Mol. Biol. 92 411–423. 10.1007/s11103-016-0521-4 [DOI] [PubMed] [Google Scholar]
- Lou Y., Baldwin I. T. (2006). Silencing of a germin-like gene in Nicotiana attenuata improves performance of native herbivores. Plant Physiol. 140 1126–1136. 10.1104/pp.105.073700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C., Jian G., Sun W. (1997). Current status, problem and countermeasure on resistance breeding to verti- cillium wilt of cotton in China. Sci. Agric. Sin. 30 58–64. [Google Scholar]
- Mejía-Teniente L., Joaquin-Ramos A., Torres-Pacheco I., Rivera-Bustamante R., Guevara-Olvera L., Rico-García E., et al. (2015). Silencing of a germin-like protein gene (CchGLP) in Geminivirus-resistant pepper (Capsicum chinense Jacq.) BG-3821 increases susceptibility to single and mixed infections by Geminiviruses PHYVV and PepGMV. Viruses 7 6141–6151. 10.3390/v7122930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmiya A. (2002). Characterization of ABP19/20, sequence homologues of germin-like protein in Prunus persica L. Plant Sci. 163 683–689. 10.1016/s0168-9452(02)00231-5 [DOI] [Google Scholar]
- Ohmiya A., Tanaka Y., Kadowaki K., Hayashi T. (1998). Cloning of genes encoding auxin-binding proteins (ABP19/20) from peach: significant peptide sequence similarity with germin-like proteins. Plant Cell Physiol. 39 492–499. 10.1093/oxfordjournals.pcp.a029396 [DOI] [PubMed] [Google Scholar]
- Ono M., Sage-Ono K., Inoue M., Kamada H., Harada H. (1996). Transient increase in the level of mRNA for a germin-like protein in leaves of the short-day plant Pharbitis nil during the photoperiodic induction of flowering. Plant Cell Physiol. 37 855–861. 10.1093/oxfordjournals.pcp.a029022 [DOI] [PubMed] [Google Scholar]
- Park H. C., Min C. K., Chan Y. P., Chung W. S., Lim C. O., Sang Y. L., et al. (2004). Pathogen- and NaCl-induced expression of the SCaM-4 promoter is mediated in part by a GT-1 box that interacts with a GT-1-like transcription factor. Plant Physiol. 135 2150–2161. 10.1104/pp.104.041442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeccam D., Patricka R., Patriciam M., Jane L. (2009). Germins: a diverse protein family important for crop improvement. Plant Sci. 177 499–510. 10.1016/j.plantsci.2009.08.012 [DOI] [Google Scholar]
- Reynolds C. R., Islam S. A., Sternberg M. J. E. (2018). EzMol: A web server wizard for the rapid visualisation and image production of protein and nucleic acid structures. J. Mol. Biol. 430 2244–2248. 10.1016/j.jmb.2018.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietz S., Bernsdorff F. E. M., Cai D. (2012). Members of the germin-like protein family in Brassica napus are candidates for the initiation of an oxidative burst that impedes pathogenesis of Sclerotinia sclerotiorum. J. Exp. Bot. 63 5507–5519. 10.1093/jxb/ers203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto A., Nishimura T., Miyaki Y., Watanabe S., Takagi H., Izumi S., et al. (2015). In vitro and in vivo evidence for oxalate oxidase activity of a germin-like protein from azalea. Biochem. Bioph. Res. Commun. 458 536–542. 10.1016/j.bbrc.2015.02.002 [DOI] [PubMed] [Google Scholar]
- Shah K., Kumar R. G., Verma S., Dubey R. S. (2001). Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci. 161 1135–1144. 10.1016/s0168-9452(01)00517-9 [DOI] [Google Scholar]
- Shetty N. P., Jørgensen H. J. L., Jensen J. D., Collinge D. B., Shetty H. S. (2008). Roles of reactive oxygen species in interactions between plants and pathogens. Eur. J. Plant. Pathol. 121 267–280. 10.1007/978-1-4020-8780-6_6 [DOI] [Google Scholar]
- Staiger D., Apel K., Trepp G. (1999). The Atger3 promoter confers circadian clock-regulated transcription with peak expression at the beginning of the night. Plant Mol. Biol. 40 873–882. [DOI] [PubMed] [Google Scholar]
- Stoilova T., Chavdarov P. (2006). Evaluation of Lentil Germplasm for disease resistance to Fusarium wilt (Fusarium oxysporum f.sp. Lentis). Pak. J. Biol. Sci. 2 394–395. [Google Scholar]
- Sultana T., Deeba F., Naz F., Rose R. J., Saqlan Naqvi S. M. (2016). Expression of a rice GLP in Medicago truncatula exerting pleiotropic effects on resistance against Fusarium oxysporum through enhancing FeSOD-like activity. Acta Physiol. Plant 38:255. [Google Scholar]
- Swart S., Logman T. J., Smit G., Lugtenberg B. J., Kijne J. W. (1994). Purification and partial characterization of a glycoprotein from pea (Pisum sativum) with receptor activity for rhicadhesin, an attachment protein of Rhizobiaceae. Plant Mol. Biol. 24:171. 10.1007/bf00040583 [DOI] [PubMed] [Google Scholar]
- Thatcher L. F., Anderson J. P., Singh K. B. (2005). Plant defence responses: what have we learnt from Arabidopsis? Funct. Plant. Biol. 32 1445–4408. [DOI] [PubMed] [Google Scholar]
- Tran P. T., Choi H., Kim S. B., Lee H. A., Choi D., Kim K. H. (2014). A simple method for screening of plant NBS-LRR genes that confer a hypersensitive response to plant viruses and its application for screening candidate pepper genes against Pepper mottle virus. J. Virol. Methods 201 57–64. 10.1016/j.jviromet.2014.02.003 [DOI] [PubMed] [Google Scholar]
- Vallelian-Bindschedler L., Mosinger E., Metraux J. P., Schweizer P. (1998). Structure, expression and localization of a germin-like protein in barley (Hordeum vulgare L.) that is insolubilized in stressed leaves. Plant Mol. Biol. 37 297–308. [DOI] [PubMed] [Google Scholar]
- Verma S., Dubey R. S. (2003). Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 164 645–655. 10.1016/s0168-9452(03)00022-0 [DOI] [Google Scholar]
- Walters D. R. (2003). Polyamines and plant disease. Phytochemistry 64 97–107. 10.1016/s0031-9422(03)00329-7 [DOI] [PubMed] [Google Scholar]
- Wang P., Sun Y., Pei Y., Li X., Zhang X., Li F., et al. (2018). GhSNAP33, a t-SNARE protein from Gossypium hirsutum, mediates resistance to Verticillium dahliae infection and tolerance to drought stress. Front. Plant. Sci. 9:896. 10.3389/fpls.2018.00896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Li F., Zhang X., Zhang Y., Hou Y., Zhang S., et al. (2011a). Purification and characterization of a CkTLP protein from Cynanchum komarovii seeds that confers antifungal activity. PLoS One 6:e16930. 10.1371/journal.pone.0016930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Zhang X., Li F., Hou Y., Liu X., Zhang X. (2011b). Identification of a UDP-glucose pyrophosphorylase from cotton (Gossypium hirsutum L.) involved in cellulose biosynthesis in Arabidopsis thaliana. Plant Cell Rep. 30 1303–1312. 10.1007/s00299-011-1042-x [DOI] [PubMed] [Google Scholar]
- Wang T., Chen X. P., Zhu F. H., Li H. F., Li L., Yang Q. L., et al. (2013). Characterization of peanut germin-like proteins, AhGLPs in plant development and defense. PLoS One 8:e61722. 10.1371/journal.pone.0061722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Zhang Z., Andersen C. H., Schmelzer E., Gregersen P. L., Collinge D. B., et al. (1998). An epidermis/papilla-specific oxalate oxidase-like protein in the defence response of barley attacked by the powdery mildew fungus. Plant Mol. Biol. 36 101–112. [DOI] [PubMed] [Google Scholar]
- Woo E. J., Dunwell J. M., Goodenough P. W., Marvier A. C., Pickersgill R. W. (2000). Germin is a manganese containing homohexamer with oxalate oxidase and superoxide dismutase activities. Nat. Struct. Biol. 7 1036–1040. [DOI] [PubMed] [Google Scholar]
- Woo E. J., Dunwell J. M., Goodenough P. W., Pickersgill R. W. (1998). Barley oxalate oxidase is a hexameric protein related to seed storage proteins: evidence from X-ray crystallography. FEBS Lett. 437 87–90. 10.1016/s0014-5793(98)01203-4 [DOI] [PubMed] [Google Scholar]
- Zambounis A. G., Paplomatas E., Tsaftaris A. S. (2008). Intergenic Spacer-RFLP analysis and direct quantification of Australian Fusarium oxysporum f. sp. vasinfectum isolates from soil and infected cotton tissues. Plant Dis. 91 1564–1573. 10.1094/PDIS-91-12-1564 [DOI] [PubMed] [Google Scholar]
- Zhang N., Guan R., Yang Y., Bai Z., Ge F., Liu D. (2017). Isolation and characterization of a Fusarium oxysporum-resistant gene LrGLP1 from Lilium regale Wilson. In Vitro Cell Dev. Bio. Plant 53 461–468. 10.1007/s11627-017-9829-2 [DOI] [Google Scholar]
- Zhang Y., Wang X., Chang X., Sun M., Zhang Y., Li W., et al. (2018). Overexpression of germin-like protein GmGLP10 enhances resistance to Sclerotinia sclerotiorum in transgenic tobacco. Biochem. Bioph. Res. Commun. 497 160–166. 10.1016/j.bbrc.2018.02.046 [DOI] [PubMed] [Google Scholar]
- Zimmermann G. (2006). The multigene family encoding Germin-like proteins of barley. Regulation and function in basal host resistance. Plant Physiol. 142 181–192. 10.1104/pp.106.083824 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.