Present clinical solutions for the functional loss or failure of tissues include implanted medical devices and tissue transplants. With some needed technical innovations and performance improvements, tissue engineering products have the potential to replace several conventional tissue repair options. The global tissue engineering market is estimated to be valued at $11.5 billion by 2022, according to Grand View Research, Inc (www.grandviewresearch.com/press-release/global-tissue-engineering-market). The initial concept of tissue engineering, comprising three integrated components 1) matrices for cell carriers, 2) isolated viable cells, and 3) signaling molecules for promoting specific cell activities was suggested by Langer and Vacanti in 1993 [1]. Tissue engineering requires scaffolds or matrices to deliver seeded cells to target tissue sites. However, to exploit biomaterials in matrices in tissue engineering and regenerative medicine, materials biocompatibility, biodegradability, inflammatory response, cellular behavior, and mechanical properties must be carefully considered [2].
Okano invented a versatile cell delivery method, matrix-free cell sheet tissue engineering exploiting a temperature-sensitive cell culture surface [3], [4]. The temperature-sensitive cell culture surface is modified by covalent attachment of a ∼20 nm thick temperature-responsive poly (N-isopropylacrylamide) (PIPAAm) layer on tissue culture polystyrene surfaces using electron beam irradiation. Cells cultured on the polymer-grafted tissue culture surface develop intrinsic cell junctions and secrete adhesive proteins at confluence, cell sheets are released from the polymer-grafted surface by small changes of temperature from 37 °C to 20 °C without any enzyme treatment [5]. Recovered cell sheets retain intact cell junctions and adhesive proteins (signaling molecules for cell activity) [6], [7]. This feature allows cell sheets to rapidly and spontaneously transplant to target tissue surfaces without supporting scaffolds, and manifest consistently greater cell survival [8]. Using autologous cell sources, cell sheet tissue engineering has treated seven human diseases including cardiac [9], [10], corneal [11], [12], esophageal [13], [14], periodontal [15], [16], middle ear [17], knee cartilage [18], and lung [19].
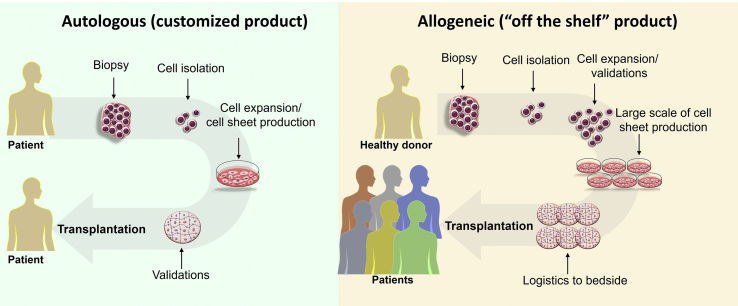
Autologous cell sourcing has no risk of graft versus host disease (GVHD) and no need for immunosuppressive therapy to prevent GVHD. However, it is high cost: products are produced in single batches on demand using a tedious manufacturing process for customized patient-specific products. Quality control is difficult: cell sourcing quality from each patient lacks control and can pose substantial challenges for autologous therapies [20]. On the other hand, allogeneic cell sourcing can produce a single large-scale batch of cells to treat significant numbers of patients and eliminate donor variability (e.g. aging and disease pathophysiologies) affecting cell quality as “off-the-shelf” cell therapy products (Fig. 2) [21]. Motivated by the compelling need to develop allogeneic cell sheets for more widespread use for tissue repair, Okano opened the cell sheet tissue engineering center at University of Utah (Utah CSTEC, USA) dedicated to allogeneic cell sheet translational applications in 2015.
Fig. 2.
An illustrator to show cell sheet production process using autologous or allogeneic cell sources.
Currently, Utah CSTEC focuses on mesenchymal stem cells (MSCs) as allogeneic cell sources. MSCs exhibit known advantages in producing diverse bioactive factors to promote tissue regeneration and modulate host immune response. Utah CSTEC possesses unique MSC sources gathered from international collaborators for potential broad use: human umbilical cord stem cells with low MHC; homogeneous human clonal bone marrow stem cells with high functionality; and liver-derived MSCs with high HGF secretion capability. MSC sheet development includes four steps: 1) careful cell characterization, 2) reliable MSC sheet preparation and harvesting/manipulation, 3) MSC sheet structural and functional characterization, and 4) reliable transplantation for therapy. In cell therapy, functional cells are the therapeutic agents. Thus, it is important to preserve cell phenotype and function (cell characterization) in manufacturing bioprocessing to ensure therapeutic efficacy of the resulting products (i.e., through intensive MSC characterization at all steps). MSC sheet preparation and harvesting/manipulation methods are optimized for specific parameters including cell seeding density, passage number, culture media, and harvesting time for each cell type to enable reproducible allogeneic cell sheets. Production of functional MSC sheets emphasizes retention of cell sheet-specific structures (i.e. cell junctions) that enhance cell–cell communication and paracrine stimuli directly affecting therapeutic efficacy (MSC sheet characterization). An important MSC sheet-specific features is sheet production of intrinsic cell adhesion proteins (matrix) shown critical for transplantation efficacy. These MSC sheet features allow rapid sheet adherence to target tissue surfaces and continual secretion of MSC-specific paracrine factors useful to tissue repair. Also, MSC sheet keeps their immunological characteristics.
Utah CSTEC seeks to develop new regenerative medicine approaches using these allogeneic MSC sheets. Utah CSTEC focuses on renal and uterine fibrotic disease treatment collaborating with Utah School of Medicine (SOM) nephrology and obstetrics-gynecology (OB/GYN) divisions (Fig. 1). Utah CSTEC cell sheet research with Utah SOM cardiothoracic surgery colleagues benefits from expert technical support from T. Shimizu at the Tokyo Women's Medical University (TWMU) to address cardiac insufficiencies using allogeneic MSC sheets (Fig. 1). Additionally, Utah CSTEC and Utah School of Dentistry colleagues have produced a submandibular gland cell sheet retaining cell–cell epithelial tight junctions and secretory granules to treat salivary gland disorders (Fig. 1). Furthermore, Utah CSTEC intends to treat articular cartilage defects with Utah SOM's orthopedics partners using allogeneic human polydactyly cell sheet. The human polydactyly cell sheets are made from pediatric polydactyly patient-derived randomized de-identified polydactyly tissue discards after surgical removal. Primary cell culture from enzyme-treated cell sources produces mostly chondrocytes. Importantly, M. Sato's group at Tokai University (Japan) has provided techniques essential for allogeneic human polydactyly cell sheet production to the Utah team (Fig. 1). This allogeneic polydactyly cell sheet project for cartilage regenerative medicine is Utah CSTEC's current model for establishing a clinical translational system, IND application for FDA regulatory approval and possible future first-in-human trials in the USA.
Fig. 1.
An illustrator to show collaborative projects of CSTEC at Utah (Utah CSTEC).
Utah CSTEC facilities in the new L.S. Skaggs Institute at the University of Utah, are supported by the College of Pharmacy. Utah CSTEC clinical translational research projects were initially supported by Utah SOM and Utah Science Technology and Research (USTAR; State of Utah) seed funds. The Utah CSTEC team management effort is anchored by D.W. Grainger, former chair of Pharmaceutics and Pharmaceutical Chemistry and current chair of Biomedical Engineering at Utah. Cell sheet science experts (K. Kim, M. Kondo and S. Kameishi-Kondo) lead Utah CSTEC allogeneic cell sheet protocol development, validation and application feasibility assessments. CSTEC clinical scientists (G. Kuramoto, M. Oka) validate new regenerative medicine approaches in several applications using the allogeneic cell sheets in preclinical models. Biomedical engineering Ph.D. students (H. Thorp, S. Bou-Ghannam, C. Dunn and N. Metzler) are working together with cell sheet science experts in all aspects of human MSC selection and profiling, MSC sheet production, characterization and preclinical validation. The international Utah CSTEC team comprising researchers and clinicians from Japan and USA is committed to realizing pioneering new human translational capabilities in regenerative medicine. Off-the-shelf allogeneic cell sheet regenerative approaches in Utah are targeted for FDA IND approval, first-in-human trials and scaled, affordable commercialization as a model center of excellence. To accelerate this mission, key collaborations with the Japanese cell sheet tissue engineering center (CSTEC) at Tokyo Women's Medical University and with M. Sato's group at Tokai University, both experienced with cell sheet translation to humans in multiple tissue regenerative approaches, are extremely valuable.
Significant unmet medical needs in regenerative medicine will only be realistically addressed using cell therapies that are reliable, off-the-shelf, commercially scalable and affordable [22], [23]. Utah CSTEC cell sheet strategies target these current cell therapy translational challenges.
Contributions
Dr. Kim drafted the main manuscript and managed the project as a corresponding author. Dr. Grainger managed the project as co-principal investigator. Dr. Okano organized and supervised the project as principal investigator.
Competing interests
Teruo Okano holds equity in CellSeed, Inc. (Japan) and is an inventor/developer designated on the patent for CellSeed's commercialized temperature-responsive culture surfaces. No other competing financial interests exist. The authors declare that they have no competing interests.
Acknowledgements
This work was supported by University of Utah Health Sciences translational research partnerships (cardiothoracic, orthopedics and OB/GYN), and the University Technology Acceleration Grant from Utah Science, Technology, and Research (USTAR) program, Utah, USA. We would like to thank T. Shimizu, T. Iwata, K. Matsuura and J. Homma at Tokyo Women's Medical University for providing technical support and training to University of Utah based students. We also appreciate M. Sato at Tokai University for providing important techniques essential for allogeneic human polydactyly cell sheet project.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Langer R., Vacanti J.P. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.O'Brien F.J. Biomaterials & scaffolds for tissue engineering. Mater Today. 2011;14(3):88–95. [Google Scholar]
- 3.Okano T., Yamada N., Okuhara M., Sakai H., Sakurai Y. Mechanism of cell detachment from temperature-modulated, hydrophilic-hydrophobic polymer surfaces. Biomaterials. 1995;16(4):297–303. doi: 10.1016/0142-9612(95)93257-e. [DOI] [PubMed] [Google Scholar]
- 4.Okano T., Yamada N., Sakai H., Sakurai Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide) J Biomed Mater Res. 1993;27(10):1243–1251. doi: 10.1002/jbm.820271005. [DOI] [PubMed] [Google Scholar]
- 5.Yamato M., Okano T. Cell sheet engineering. Mater Today. 2004;7(5):42–47. [Google Scholar]
- 6.Kushida A., Yamato M., Konno C., Kikuchi A., Sakurai Y., Okano T. Decrease in culture temperature releases monolayer endothelial cell sheets together with deposited fibronectin matrix from temperature-responsive culture surfaces. J Biomed Mater Res. 1999;45(4):355–362. doi: 10.1002/(sici)1097-4636(19990615)45:4<355::aid-jbm10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Yamato M., Utsumi M., Kushida A., Konno C., Kikuchi A., Okano T. Thermo-responsive culture dishes allow the intact harvest of multilayered keratinocyte sheets without dispase by reducing temperature. Tissue Eng. 2001;7(4):473–480. doi: 10.1089/10763270152436517. [DOI] [PubMed] [Google Scholar]
- 8.Sekine H., Shimizu T., Dobashi I., Matsuura K., Hagiwara N., Takahashi M. Cardiac cell sheet transplantation improves damaged heart function via superior cell survival in comparison with dissociated cell injection. Tissue Eng. 2011;17(23–24):2973–2980. doi: 10.1089/ten.tea.2010.0659. [DOI] [PubMed] [Google Scholar]
- 9.Sawa Y., Miyagawa S., Sakaguchi T., Fujita T., Matsuyama A., Saito A. Tissue engineered myoblast sheets improved cardiac function sufficiently to discontinue LVAS in a patient with DCM: report of a case. Surg Today. 2012;42(2):181–184. doi: 10.1007/s00595-011-0106-4. [DOI] [PubMed] [Google Scholar]
- 10.Sawa Y., Yoshikawa Y., Toda K., Fukushima S., Yamazaki K., Ono M. Safety and efficacy of autologous skeletal myoblast sheets (TCD-51073) for the treatment of severe chronic heart failure due to ischemic heart disease. Circ J. 2015;79(5):991–999. doi: 10.1253/circj.CJ-15-0243. [DOI] [PubMed] [Google Scholar]
- 11.Nishida K., Yamato M., Hayashida Y., Watanabe K., Maeda N., Watanabe H. Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation. 2004;77(3):379–385. doi: 10.1097/01.TP.0000110320.45678.30. [DOI] [PubMed] [Google Scholar]
- 12.Nishida K., Yamato M., Hayashida Y., Watanabe K., Yamamoto K., Adachi E. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351(12):1187–1196. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- 13.Ohki T., Yamato M., Ota M., Takagi R., Murakami D., Kondo M. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology. 2012;143(3):582–588 e2. doi: 10.1053/j.gastro.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi N., Isomoto H., Kobayashi S., Kanai N., Kanetaka K., Sakai Y. Oral epithelial cell sheets engraftment for esophageal strictures after endoscopic submucosal dissection of squamous cell carcinoma and airplane transportation. Sci Rep. 2017;7(1):17460. doi: 10.1038/s41598-017-17663-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwata T., Yamato M., Washio K., Ando T., Okano T., Ishikawa I. Cell sheets for periodontal tissue engineering. Current Oral Health Reports. 2015;2(4):252–256. [Google Scholar]
- 16.Iwata T., Yamato M., Washio K., Yoshida T., Tsumanuma Y., Yamada A. Periodontal regeneration with autologous periodontal ligament-derived cell sheets - a safety and efficacy study in ten patients. Regen Ther. 2018:938–944. doi: 10.1016/j.reth.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto K., Yamato M., Morino T., Sugiyama H., Takagi R., Yaguchi Y. Middle ear mucosal regeneration by tissue-engineered cell sheet transplantation. NPJ Regen Med. 2017;26 doi: 10.1038/s41536-017-0010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ebihara G., Sato M., Yamato M., Mitani G., Kutsuna T., Nagai T. Cartilage repair in transplanted scaffold-free chondrocyte sheets using a minipig model. Biomaterials. 2012;33(15):3846–3851. doi: 10.1016/j.biomaterials.2012.01.056. [DOI] [PubMed] [Google Scholar]
- 19.Kanzaki M., Takagi R., Washio K., Kokubo M., Yamato M. Bio-artificial pleura using an autologous dermal fibroblast sheet. NPJ Regen Med. 2017;226 doi: 10.1038/s41536-017-0031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaiser A.D., Assenmacher M., Schroder B., Meyer M., Orentas R., Bethke U. Towards a commercial process for the manufacture of genetically modified T cells for therapy. Cancer Gene Ther. 2015;22(2):72–78. doi: 10.1038/cgt.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rachel Haddock S.L.-G., Lumelsky Nadya, McFarland Richard, Roy Krishnendu, Saha Krishanu, Zhang Jiwen. 2017. Manufacturing cell therapies: the paradigm shift in Health care of this century national academy of medicine; pp. 1–13. [Google Scholar]
- 22.Abou-El-Enein M., Bauer G., Reinke P. The business case for cell and gene therapies. Nat Biotechnol. 2014;32(12):1192–1193. doi: 10.1038/nbt.3084. [DOI] [PubMed] [Google Scholar]
- 23.Hettle R., Corbett M., Hinde S., Hodgson R., Jones-Diette J., Woolacott N. The assessment and appraisal of regenerative medicines and cell therapy products: an exploration of methods for review, economic evaluation and appraisal. Health Technol Assess. 2017;21(7):1–204. doi: 10.3310/hta21070. [DOI] [PMC free article] [PubMed] [Google Scholar]