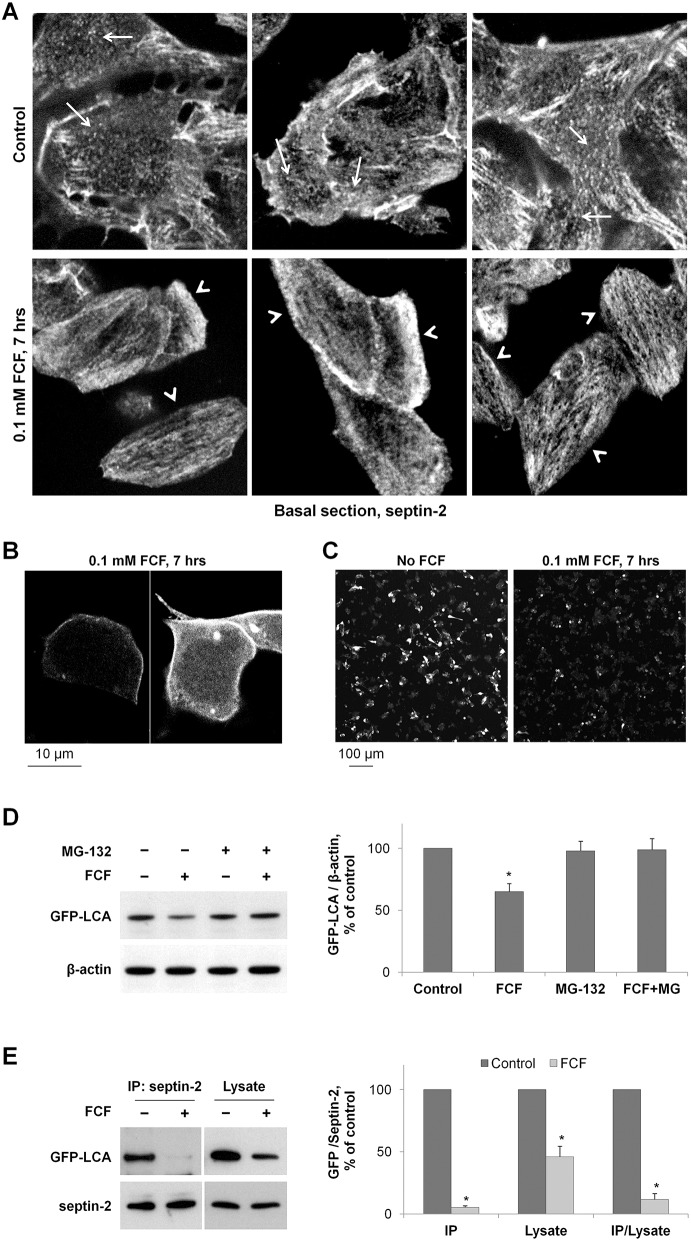
Fig. 6.
Both downregulation of septin-2 and disruption of septin organization by FCF increase LCA degradation. (A) FCF disrupts the ring-like and punctuated structures of septin 2 (arrows) and induces the formation of elongated filaments (arrowheads) in SiMa cells. (B) High-resolution/high-magnification confocal microscopy images of non-differentiated GFP-LCA-expressing SiMa cells exposed to 100 µM FCF showing increased cytoplasmic localization of LCA and relatively even/homogeneous distribution of LCA along the plasma membrane. (C) Non-differentiated SiMa cells plated with similar density were transfected with GFP–LCA and 14 h later were incubated with or without FCF for 7 h. Low magnification confocal microscopy images of these cells show a substantial decrease in GFP fluorescence in FCF-exposed cells. (D) Disruption of septin organization by FCF induces degradation of GFP–LCA. Non-differentiated cells expressing GFP–LCA were incubated with cycloheximide in the absence or in the presence of 100 µM FCF or 20 µM MG-132. The amount of LCA and β-actin in total cell lysates was determined by immunoblotting. Quantification of the results indicates that exposure of cells to FCF decreases the amount of mature GFP–LCA in the absence, but not in the presence, of the degradation inhibitor MG-132. (E) FCF impairs the interaction between septin-2 and LCA. Septin-2 immunoprecipitation was performed after incubation of GFP–LCA-expressing cells with or without FCF for 24 h, and the amount of both septin-2 and GFP–LCA was determined by immunoblotting in immunoprecipitated fraction and total cell lysates. Densitometry quantification of the results shows that FCF decreases the amount of GFP–LCA in the septin-2 immunoprecipitated fractions more dramatically than in cell lysates, indicating its disruptive effect on septin-2-LCA interaction. Results are mean±s.d. (n = 3); *P<0.01 compared with control (no inhibitors), Student's t-test.