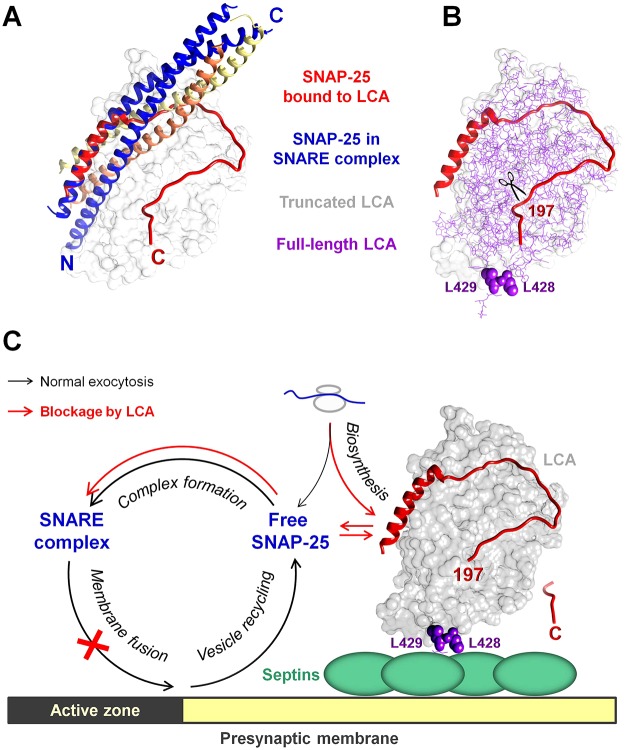
Fig. 7.
A postulated model showing that stabilization of LCA by septins at the presynaptic membrane results in persistent cleavage of the free pool of SNAP-25 and prolonged inhibition of neurotransmission. (A) Superposition of high resolution structures of SNAP-25 within a SNARE complex (PDB ID 1KIL) and SNAP-25 bound to LCA (PDB ID 1XTG) showing significant differences in the conformation of the C-terminal domain of SNAP-25 in these two protein complexes. The tight interaction of the C-terminal domain of SNAP-25 with its N-terminal domain and with other SNAREs, syntaxin (yellow) and synaptobrevin (orange), within the SNARE complex is not compatible with LCA-catalyzed cleavage of SNARE-complex-bound SNAP-25. (B) Superposition of high-resolution structures of a truncated LCA (gray surface) bound to the C-terminal core domain of SNAP-25 (PDB ID 1XTG) with a full-length LCA (PDB ID 2W2D, thin purple lines) shows no hindrance of L428 and L429 (purple spheres) by bound SNAP-25, indicating that the dileucine-mediated interaction of LCA with septins is permissive for the LCA proteolytic activity. (C) A putative model showing that the dileucine-mediated interaction with septin filaments tethers LCA to the presynaptic membrane in the vicinity of an active zone, where it cleaves SNAP-25 monomers that are either newly synthesized or released from SNARE complexes after synaptic vesicle fusion. After the cleavage of the nine C-terminal amino acid residues by LCA, SNAP-25197 forms SNARE complexes that are not competent for exocytosis, resulting in a blockage of synaptic vesicle fusion and neurotransmitter release. Association of LCA with septins protects LCA from intracellular degradation, providing an explanation for the remarkable longevity of proteolytic and, probably, paralytic effects of LCA in motor neurons.