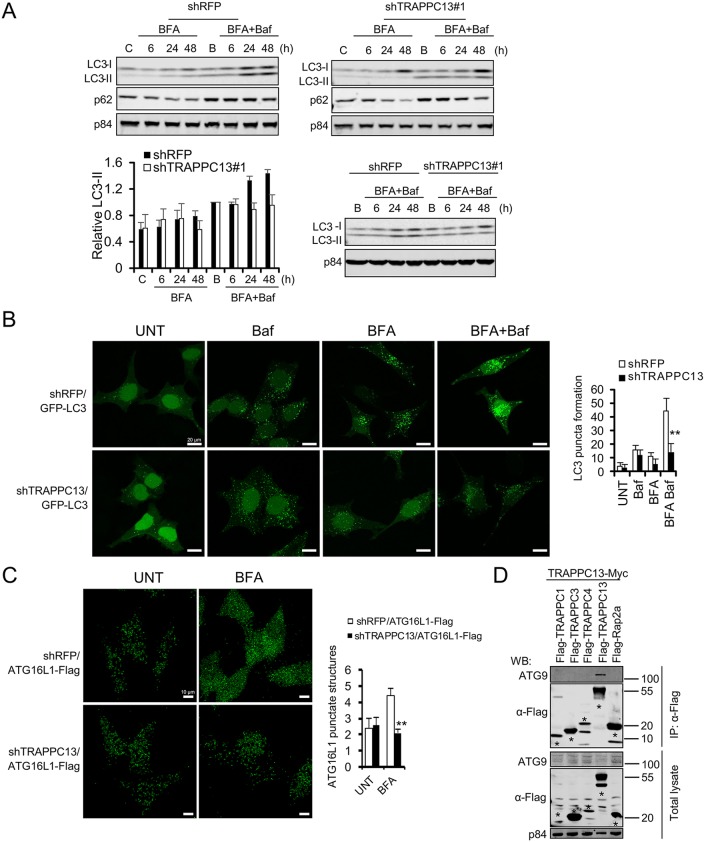
Fig. 5.
Loss of TRAPPC13 leads to impaired autophagic flux in response to BFA treatment. (A) Stable shTRAPPC13 or shRFP knockdown HeLa cells were treated with 12.5 ng/ml BFA for the indicated times in the presence of 20 nM bafilomycin for the last 3 h of incubation. Untreated control cells were incubated in DMEM for 6 h in the presence (labelled as B) or absence (labelled as C) of bafilomycin A1 (Baf) for the last 3 h of incubation. Protein lysates were resolved by SDS-PAGE and immunoblotted with the indicated antibodies. Quantification of relative LC3 II levels was performed as described in the Materials and Methods. Samples shown in the upper two western blot panels were additionally run and evaluated on the same gel as displayed in the lower western blot panel to confirm reduced LC3-II levels in TRAPPC13 knockdown cells compared with control shRFP cells. Data are representative of three independent experiments. (B) HeLa cells stably expressing GFP-LC3 and transduced with shRFP or shTRAPPC13 hairpins were incubated with DMEM in the absence or presence of 20 ng/ml BFA for 24 h with or without Baf for the last 3 h of incubation. The expression of GFP-LC3 was examined by confocal microscopy. LC3 dots in cells were measured as described in the Materials and Methods. Representative quantification results from two independent experiments are shown. **P<0.01 (two-tailed Student's t-test). (C) HeLa cells stably expressing ATG16L1-Flag and transduced with shRFP or shTRAPPC13 hairpins were treated with BFA for 24 h or left untreated. ATG16L1-Flag accumulation was assessed by IF using confocal microscopy. Representative quantification results from two independent experiments are shown. ** P<0.01 (two-tailed Student's t-test). (D) TRAPPC13 interacts with ATG9. The indicated proteins were transiently expressed in HEK293T cells before Flag IP and blotting for endogenous ATG9.