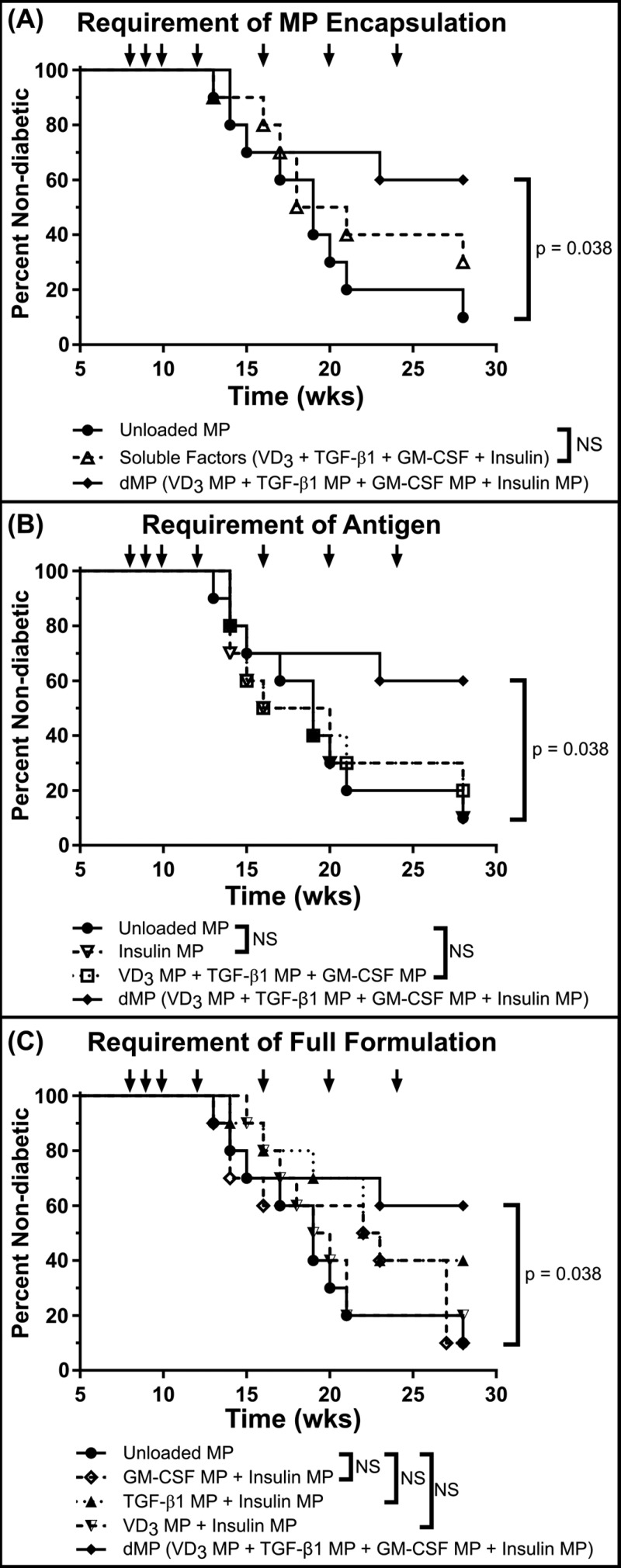
Figure 6.
dMP administration prevents diabetes onset in NOD mice. A cohort of 8-week-old NOD mice (n = 10/group) were injected at a subcutaneous site anatomically proximal to the pancreas with the described MP formulations over 16 weeks. Animals received MP injections (arrows) once a week for the first 3 weeks (8, 9, and 10 weeks of age) and a booster injection once monthly thereafter for 4 months (12, 16, 20, and 24 weeks of age). Unloaded MPs, a soluble bolus of factors without MPs, and omission of factors were investigated. When a factor-loaded MP was omitted, unloaded MPs were delivered to deliver an equivalent PLGA mass. Animals were monitored weekly until week 28, and mice were considered diabetic when blood glucose levels were ≥240 mg/dL on 2 consecutive days. The full dMP (solid line with solid tilted square, VD3/TGF-β1/GM-CSF/insulin MPs) and unloaded MPs groups were replotted alongside different experimental groups to highlight the requirement of MP encapsulation (A), antigen (B), and the full dMP formulation (C) in order to see maximum therapeutic effect. Survival data are fit using the Kaplan–Meier nonparametric survival analysis model, and statistical analysis was performed via log-rank test (Mantel–Cox method). Statistical significance was not realized when accounting for multiple comparisons via Bonferroni correction, as the study was not powered to resolve this large number of groups. However, pairwise comparison between survival curves of mice that received the dMP and mice that received unloaded MPs resulted in a P-value of <0.05, suggesting a difference between treatments.