Fig. 4.
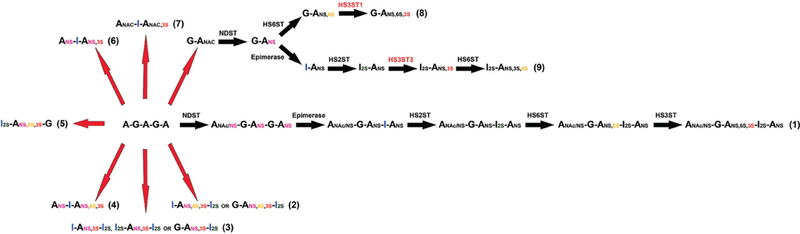
Different 3-O-sulfated heparin sequences found in animals and produced chemoenzymatically. (1) A minimum sequence of heparin (pentasaccharide) involved in AT-binding. It has been proposed that the pentasaccharide is synthesized according to the classical biosynthetic pathway, in which the enzymatic events happen through a hierarchical sequence, as described by Lindahl, 1977.3 (2 and 3) Sequences present in heparin described in Lindahl, 1994.24 (4) Heparin sequence found in clams that does not correlate with affinity for AT.17 (5) Sequence described in shrimp L. vannameithat has negligible anticoagulant activity despite its high affinity for AT and unusually higher proportion of 3-O-sulfated residues.19 (6 and 7) Scheme for 3-O-sulfated heparin sequences described by this study. The starting material for chemical modifications was porcine intestinal mucosa heparin, the schemes show iduronate rather than glucuronate once heparin does have significant higher proportions of iduronate. (8 and 9) Different oligosaccharide substrates required by HS3ST isoforms. While HS3ST3 must precede the 6-O-sulfation to generate the I2S-ANS3S6S, HS3ST1 can only work after the 6-O-sulfation step.15
