Abstract
Motivation has played an integral role in understanding personality development. Two motivational systems, one associated with seeking reward (approach motivation) and one associated with avoidance of threat (avoidance motivation), have been theorized to represent individual differences in behavioral responses to the environment. However, contextual factors, particularly those with a high degree of novelty, ambiguity, and unpredictability, may simultaneously activate both systems, thereby causing approach-avoidance conflict. The resulting behavior, commonly called inhibition, is characterized by an inability to engage in motivated, goal-directed behavior and is theorized to reflect a core component of anxiety. A form of inhibition observed in childhood, behavioral inhibition (BI), is a relatively stable temperamental profile characterized by negative affect in response to unfamiliar and unpredictable contexts and is a risk factor for anxiety. Our review draws from findings in clinical and cognitive neuroscience to argue that BI reflects an increased sensitivity of both approach and avoidance motivational systems, thereby increasing the likelihood of approach-avoidance conflict within the context of unfamiliar or unpredictable stimuli and environments. Such motivational conflict activates neural systems associated with conflict monitoring, which leads to increases in arousal (e.g., sympathetic nervous system activity) and onlooking behavior, two commonly observed characteristics of childhood BI.
1. Introduction
Numerous theories attempt to account for the vast presentations of individual differences in temperament and personality in humans (Cattell, 1957; Digman, 1990; Eysenck, 1963; Goldberg, 1993; Gray, 1981; John & Srivastava, 1999; Rothbart, 2007). One orientation to personality development emphasizes the role of motivated behavior in shaping stable patterns of behavioral response to the environment (Beauchaine & Zisner, 2017; Berridge, 2004; Fowles, 1988; Gray, 1981; Mackintosh, 1974). Among these theories, it is typically agreed upon that two independent, neurobiological-based motivational systems contribute to goal-directed behavior (Corr, 2004; Gray & McNaughton, 2003). The approach-related system is associated with an organism’s motivation to seek and obtain rewarding stimuli and environments. In contrast, the avoidance-related system is associated with active behaviors to avoid unpleasant stimuli and environments. Research in both human and animal neuroscience largely confirms that distinct neural systems guide approach and avoidance motivation, which we describe in detail later (Berridge & Robinson, 2003; Davis, Walker, Miles, & Grillon, 2009; Gray & McNaughton, 2003; LeDoux, 1995).
Although approach and avoidance motivation are relatively distinct, they are often coactivated by environmental factors (Corr, 2004; Gray & McNaughton, 2003). Contexts that elicit both motivational systems typically are those that are highly novel, ambiguous, and unpredictable (Corr, 2004; Gray & McNaughton, 2003; Grillon, 2002; Grillon, 2008). These contextual factors are perceived to comprise the possibilty of both reward and punishment and may require basic cost-benefit comparison before approach or avoidance behaviors are adopted (Aupperle & Paulus, 2010). For example, a novel but ambiguous situation could either provide rewards or cause harm. The relative safety benefits of avoiding the novel situation must be weighed against the possibility of sacrificing potential rewards. In such instances, there is conflict between approach and avoidance motivational systems, because both systems are activated (Gray, 1982; Gray & McNaughton, 2003). Recent findings in affective neuroscience have found that contextual factors known to cause approach-avoiandce conflict, such as unpredictability, increase activation of both approach and avoidance motivational systems (Gorka, Nelson, Pha, & Shackman, 2016; Grillon, 2008; Shankman et al., 2014), as well as increase actvation of systems that detect conflict (Jackson, Nelson, & Hajcak, 2016). Critically, a high level of approach-avoidance conflict is theorized to result in the inhibition of behavior (Gray, 1981; Gray & McNaughton, 2003). Here we refer to the concept of inhibition as stopping/interrupting goal-directed behavior in contexts that elicit approach-avoidance conflict.
The concept of inhibition has been described extensively by two relatively distinct psychological disciplines. Jeffrey Gray, a clinical psychologist who is best known for his theory of motivation in contributing to personality (Corr, 2004; Gray & McNaughton, 2003), described inhibition as the behavior that results from approach-avoidance conflict (Gray & McNaughton, 2003). Inhibition has also been described by Jerome Kagan, a developmental psychologist whose career in part focused on understanding the neurobiology of temperamental inhibition in toddlers and young children (Coll, Kagan, & Reznick, 1984; Kagan, Reznick, Clarke, Snidman, & Garcia-Coll, 1984). When discussing these theoretical constructs, we refer to the behavioral inhibition system (BIS) as the conflict system described by Gray and behavioral inhibition (BI) as the temperamental profile described by Kagan.
A growing body of research is characterizing the mechanisms associated with conflict processing and subsequent behavioral adaptations following motivational conflict (Aupperle, Melrose, Francisco, Paulus, & Stein, 2015; Aupperle & Paulus, 2010). Research in cognitive neuroscience has also extensively examined the neural systems dedicated to conflict detection and resolution (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Ullsperger, Danielmeier, & Jocham, 2014; Wessel, 2018; Wessel, Danielmeier, Morton, & Ullsperger, 2012). These systems are thought to detect conflict and cause momentary inhibition of behavior as a way to improve future goal-directed behavior (Wessel, 2018). Similarly, both Gray and Kagan often drew upon findings in animal and human neuroscience in their quest to understand the biological basis of inhibition (Gray & McNaughton, 2003; Kagan, Reznick, & Snidman, 1988). However, to the best of our knowledge, little work has integrated Kagan and Gray’s views on inhibition (Morgan, 2006), particularly as it is related to approach and avoidance motivational systems and approach-avoidance conflict. Moreover, such work has yet to be fully integrated with the more recent cognitive neuroscience of conflict processing.
In this review, we explore the degree to which Kagan’s view of inhibition during childhood (i.e., childhood BI) can be characterized by Gray’s theory of motivational systems, personality and anxiety. Specifically, we focus on systems related to the motivation to engage in approach-related social behavior in childhood (e.g., playing with an unfamiliar peer) and to the motivation to engage in avoidance-related social behavior in childhood (e.g., leaving an unpleasant social situation). We examine how approach-avoidance conflict is associated with BI, which we argue results in an inability to engage in flexibly controlled, goal-directed behavior (i.e., inhibition), particularly in novel, ambiguous, or unpredictable contexts (Asendorpf, 1991; Coplan, Rubin, Fox, Calkins, & Stewart, 1994; Rubin, 2014; Rubin, Coplan, & Bowker, 2009). We also integrate such theory with more recent work from the field of cognitive neuroscience.
2. Motivational Systems Associated with Inhibition
In his original model, Gray (1981, 1982) postulated that motivational systems for approach and avoidance underlie individual differences in personality. Unlike Kagan’s emphasis on early temperament differences, Gray’s model of personality focused on the neurobiology of motivational systems as it relates to personality (Gray, 1982), and later, how conflict between these motivational systems produces anxiety (Gray & McNaughton, 2003). As a rebuttal to Eysenck’s theory of personality (Eysenck, 1963), Gray used findings from animal learning and emerging neuroscience research to argue that behavior is largely characterized by two independent motivational systems. As defined by Gray, the behavioral approach system (BAS) mediates motivational appetitive and approach behavior that is sensitive to receiving rewards. In contrast, Gray originally formulated the BIS to mean a motivational system that is sensitive to punishment and nonrewards, which reflects both avoidance behavior (e.g., fleeing from threat) and inhibition of behavior (i.e., abrogation of behavior and environmental scanning). Gray argued that individual differences in personality are shaped by differences in these two independent systems (Gray, 1982).
One prominent critique of Gray’s original model pointed to the difficulty in distinguishing biological systems that involve mediation of active avoidance rather than inhibition of behavior (Smillie, Pickering, & Jackson, 2006). In an updated model that leveraged more-recent insights in neuroscience (D. C. Blanchard & Blanchard, 1972; R. J. Blanchard, Yudko, Rodgers, & Blanchard, 1993). Gray clarified that what was originally referred to as the singular BIS is actually better characterized as two separate, though interrelated, systems: the fight-flight-freeze system (FFFS) and the BIS (Gray & McNaughton, 2003). Thus, the updated and most recent version of Gray’s model, reinforcement sensitivity theory (RST), includes three separate systems: the original BAS, the newly defined FFFS, and the redefined BIS (Gray & McNaughton, 2003; Smillie et al., 2006). Gray’s revised RST model describes FFFS as a motivational system characterized by behavior (e.g., escaping, aggression, freezing) that is driven by basic fear circuitry, which serves to actively defend the organism from threat. Thus, in Gray and McNaughton’s (2003) RST model, FFFS bears the closest resemblance to BIS as it was defined in Gray’s original theory.
In contrast, the BIS, as it is now defined within the RST, is specifically related to the resulting conflict that occurs when FFFS and BAS are coactivated. Gray and McNaughton (2003) theorized that the coactivation of FFFS and BAS is most likely to occur within contexts that involve a high degree of unfamiliarity or unpredictability. For example, a novel or unfamiliar stimulus could be rewarding in itself or may lead to the possibility of reward (Horvitz, 2000), but could also be harmful or threatening (D. C. Blanchard, Griebel, Pobbe, & Blanchard, 2011). In such situations of both high approach and avoidance motivation, the BIS is theorized to inhibit one’s behavior and increase attention to the environment, as well as increase physiological arousal (e.g., increases in sympathetic nervous system activity) in order to resolve the apparent motivational conflict through further processing of contextual cues/demands. Gray examined the neurobiology associated with these systems to understand psychopathology and personality, suggesting that the BIS is the core component in the presentation of anxious behavior (Clark & Watson, 1991; Corr, 2004; Gray & McNaughton, 2003).
It is important to note that inhibition of behavior and freezing behavior reflect different motivational processes (Gray & McNaughton, 2003). Freezing behavior is a defensive behavioral strategy that occurs when an animal encounters a proximal threatening stimulus (e.g., predator) and is often the resulting defensive behavioral response when there is little likelihood of escape (D. C. Blanchard et al., 2011; R. J. Blanchard & Blanchard, 1969). In contrast, behavioral inhibition, as defined by Gray, involves the abrogation of goal-directed behavior when encountering unpredictable or unfamiliar stimuli or contexts (Gray & McNaughton, 2003), resulting in environmental scanning and risk-assessment behaviors (D. C. Blanchard et al., 2011; R. J. Blanchard & Blanchard, 1969; Gray & McNaughton, 2003). One important risk-assessment behavior, known as stretched attend posture, is characterized by stretching of the body while facing or slowly moving toward a stimulus with the purpose of gaining more information about the stimulus (Grant & Mackintosh, 1963). Stretched attend posture bears strong resemblance to onlooking behavior observed in children with BI (Asendorpf, 1990b, 1991), which is characterized by careful observation of peers without engaging in play.
Developmental psychologists have long been interested in understanding early developmental predispositions to responding to the environment, which is commonly referred to as temperament (Kagan et al., 1984; Rothbart, 1981, 1986; Thomas & Chess, 1977). Kagan and colleagues observed a behavioral characteristic among a group of toddlers who presented with negative affect in response to novelty and unfamiliar contexts or people, which they termed behavioral inhibition to the unfamiliar (Coll et al., 1984; Kagan et al., 1984; Kagan, Reznick, & Snidman, 1986). Subsequent research has found that approximately 10% - 15% of children exhibit stable BI in unfamiliar contexts throughout childhood (Coll et al., 1984; Fox, Henderson, Rubin, Calkins, & Schmidt, 2001; Kagan et al., 1986). Drawing from rodent models of fear learning and anxiety, Kagan and colleagues (1984, 1986) postulated that BI in toddlers closely resembled the cross-species behavior of freezing during threat detection (R. J. Blanchard & Blanchard, 1969; LeDoux, 1995). However, we argue that childhood behaviors associated with inhibition, such as onlooking behavior (Asendorpf, 1990b, 1991), more closely resemble the stretched attend posture in animals when threat is not clearly defined (D. C. Blanchard et al., 2011; R. J. Blanchard & Blanchard, 1969; Gray & McNaughton, 2003). Similar to Gray’s suggestion that inhibition is associated with increased physiological arousal, it has been found that BI is characterized by increased sympathetic nervous system activity (Kagan et al., 1988).
Given the role of the limbic system, including the amygdala, in the processing of threat (D. C. Blanchard & Blanchard, 1972; R. J. Blanchard et al., 1993; LeDoux, 1995), Kagan postulated that BI may be related to increased reactivity of structures within the limbic system, such as the amygdala (Kagan et al., 1988). Indeed, a number of research findings confirm that the limbic system plays a role in predicting variations in BI (Barker, Reeb-Sutherland, et al., 2015; Barker, Reeb-Sutherland, & Fox, 2014; Blackford, Allen, Cowan, & Avery, 2013; Clauss et al., 2014; Perez-Edgar et al., 2007; Schmidt & Fox, 1998; Schmidt, Fox, Schulkin, & Gold, 1999; Schwartz et al., 2012; Schwartz, Wright, Shin, Kagan, & Rauch, 2003).
Observational studies of social behavior in children similarly suggest that approach-avoidance conflict elicits inhibition (Asendorpf, 1990a, 1991; Coplan et al., 1994; Rubin, Burgess, & Hastings, 2002; Rubin, Cheah, & Fox, 2001). Asendorpf (1990a) argued that the development of social behavior can similarly be classified according to independent motivational systems that guide motivation to approach peers (i.e., social approach) and the motivation to avoid peers (i.e., social avoidance). Using these two orthogonal systems, Asendorpf (1990a) categorized socialization behaviors of young children who exhibit difficulty interacting with peers into three distinct groups. First are unsociable children, who primarily exhibit low approach motivation and whose lack of peer interactions is primarily driven by a lack of social interest. Second, in contrast, are socially withdrawn children, who are characterized by high avoidance motivation and whose lack of peer interactions is driven by active avoidance of social contexts. Finally, shy children are characterized by both high approach motivation and high avoidance motivation, resulting in a number of inhibited behaviors (e.g., onlooking behavior) during peer interactions and intermediary play behaviors with peers (e.g., parallel play). A larger body of literature has confirmed that shyness, or the related concept of social reticence (Coplan et al., 1994), is distinguishable from unsociable and socially withdrawn behavior profiles (Coplan et al., 1994; Rubin, Bukowski, & Parker, 1998; Rubin & Mills, 1988). Critically, Asendorpf’s (1990a) definition of shyness, or the related notion of social reticence (Coplan et al., 1994), suggests the behavioral characteristics are associated with the temperament of BI (Asendorpf, 1991).
Although there is no clear link in the early literature, it is important to note the similarities between Kagan’s seminal work on childhood BI and Gray’s work on the BIS and the neurobiological correlates of anxiety (Gray, 1981, 1982; Gray & McNaughton, 2003). It appears that use of the same term, behavioral inhibition, at least originally was only a coincidence and that the use of the same term was not meant to refer to the same construct. Whereas Kagan’s use of inhibition arose from the desire to explain approach-avoidance behavior in young children (Kagan, Reznick, & Snidman, 1987), Gray’s use of the term was primarily intended to address the neurobiology of anxiety in relation to motivation (Corr, 2004; Gray & McNaughton, 2003). Nonetheless, as we will argue, it appears that this coincidence was quite fortuitous, given that a wide body of research has found that BI is associated with increased risk for anxiety in adolescence and adulthood (Biederman et al., 2001; Chronis-Tuscano et al., 2009; Clauss & Blackford, 2012; Hirshfeld et al., 1992; Kagan & Snidman, 1999), suggesting commonalities between Kagan’s and Gray’s theories with regard to behavioral characteristics of inhibition.
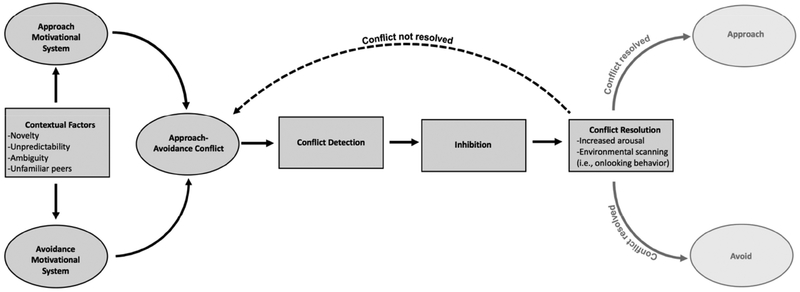
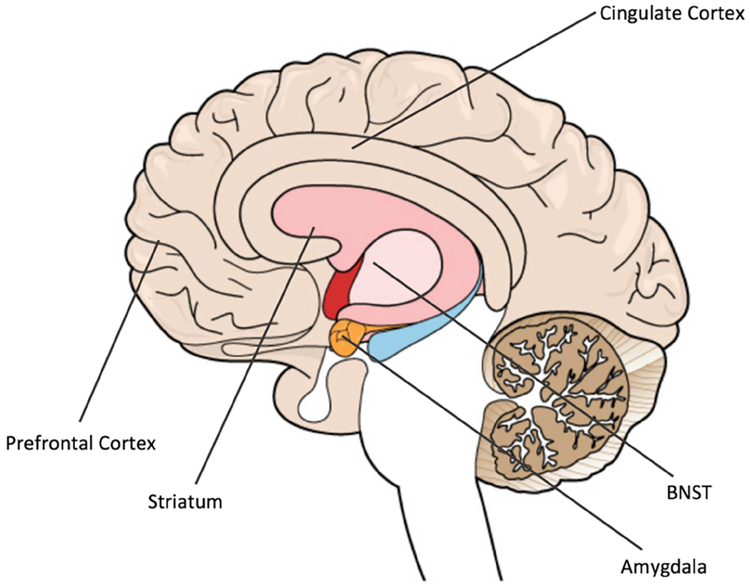
Figure 1 presents our model of how activation of motivational systems can result in childhood BI. The figure draws heavily from theories of the relation between BIS and approach- avoidance conflict as described by Gray & McNaughton (2003). Here, approach motivation is strongly driven by neural regions that mediate reward processing, such as the striatum, a small structure in the basal ganglia that contains numerous dopaminergic connections with the neocortex (see Figure 2; Balleine, Delgado, & Hikosaka, 2007; Berridge, 2004; Berridge, Robinson, & Aldridge, 2009; Delgado, Nystrom, Fissell, Noll, & Fiez, 2000; Schultz, 2007). In contrast, avoidance motivation is primarily a mechanism for harm reduction (e.g., detecting and fleeing from threat) that is mediated by processing in the amygdala (LeDoux, 2014; Shackman & Fox, 2016), as well as by an adjacent neural structure called the bed nucleus of the stria terminalis (BNST; see Figure 2), which mediates sustained arousal to threat, particularly when threat is not well defined (Avery, Clauss, & Blackford, 2016; Davis, 1998; Davis et al., 2009). Approach-avoidance conflict is the result of simultaneous activation of approach and avoidance motivational systems, which typically occurs in contexts with a high degree of novelty, ambiguity, and unpredictability, in that these contexts are perceived as both potentially rewarding and potentially punishing (Aupperle & Paulus, 2010; Gray & McNaughton, 2003; Kagan et al., 1984; Kagan et al., 1987). Social situations, particularly with unfamiliar peers or adults, are likely to elicit approach-avoidance conflict in behaviorally inhibited children.
Figure 1.
Theoretical depiction of the systems underlying the presentation of childhood behavioral inhibition.
Figure 2.
Neural regions which mediate approach motivation (striatum), avoidance motivation (amygdala and BNST), and approach-avoidance conflict (prefrontal and cingulate cortex). BNST: bed nucleus of the stria terminalis.
When approach-avoidance conflict occurs in response to contextual factors, it is detected by the conflict monitoring system, whose primary function is to support goal-directed behavior by detecting deviations from expected behavioral outcomes (Holroyd & Yeung, 2012; Yeung, Botvinick, & Cohen, 2004). The conflict monitoring system is believed to be primarily mediated by neocortical regions, such as the anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), and prefrontal cortex (PFC), all of which interface with both the approach and avoidance motivational systems (see Figure 2; Botvinick et al., 2001; Holroyd & Coles, 2002; Holroyd & Yeung, 2012).
Following the detection of conflict, inhibition occurs (i.e., abrogation of goal-directed behavior). Recent cognitive neuroscience research demonstrates that conflict detection leads to an orienting response and the momentary inhibition of behavior (Wessel, 2018). Immediately following inhibition, conflict resolution strategies take place, such as increases in psychological arousal (e.g., increased sympathetic nervous activity), as well as the occurrence of environmental scanning (i.e., onlooking behavior in children, analogous to stretched attend posture in animals), which serve the purpose of learning more about the stimulus/environment to resolve the motivational conflict (Gray & McNaughton, 2003). If conflict is resolved, behavior is then guided primarily by either of the two motivational systems (e.g., if approach systems is predominately activated then approach behavior occurs). However, if conflict is not resolved, such continued approach-avoidance conflict is detected, leading to continued inhibition.
Our theoretical model of childhood BI presented in Figure 1 leads to at least two novel hypotheses. Given that we suggest childhood BI arises directly from enhanced conflict detection prior or during approach-avoidance conflict, BI could be the ultimate result of early differences in both approach and avoidance activity, or an imbalance between these systems (Aupperle & Paulus, 2010). As such, our first hypothesis is that children characterized by BI would exhibit elevated baseline activity of both approach and avoidance motivational systems. Due to the heightened baseline activity of both motivational systems, children characterized by BI are more likely to experience approach-avoidance conflict when encountering contexts that coactivate both systems (e.g., novelty, ambiguity, unpredictability), as such contexts further increase both approach and avoidance motivation.
A second hypothesis, though not mutually exclusive to our first hypothesis, is that BI is characterized by increased sensitivity of approach-avoidance conflict rather than the sensitivity of the motivational systems. Kagan (Kagan, Reznick, & Snidman, 1986), as well as other authors (Fox et al., 2005) have emphasized that sensitivity to conflict is central to BI, and perhaps even the starting point, where increased sensitivity to conflict leads to downstream influences on behavior. Indeed, there is evidence to suggest that BI is characterized by enhanced processing of novelty (Marshall, Reeb, & Fox, 2009). Here, BI is characterized by typical activation of approach and avoidance motivation when contextual factors predominately activate one system or the other (e.g., threat is clearly defined and predictable activating only the avoidance system, or reward is predictable and clearly defined only activation the approach system). However, when contexts contain high degree of novelty, ambiguity, or unpredictability, increased sensitivity of the conflict detection system leads to greater likelihood of inhibition. In the following sections, we explore underling neurobiology of approach and avoidance motivation and motivational conflict in BI and review how contextual factors modulate the activity of these systems.
Although Gray viewed the approach and avoidance systems as largely independent (Gray & McNaughton, 2003), we take a more nuanced view of the underlying neurobiology that support approach and avoidance motivation. Specifically, we acknowledge that neural regions mediating approach motivation may be activated during avoidance motivation, and vice versa, suggesting that multiple systems may contribute to motivation and learning (Salamone & Correa, 2012). As one example, emerging work has found that different dopamine receptors within the striatum are activated by learning to avoid or approach. Specifically, phasic bursts of dopamine in the striatum are related to learning to initiate motor movements (i.e., “Go” signal; Frank, 2005; Frank, Seeberger, & O’Reilly, 2004). In contrast, dips in phasic dopamine cause an increase in learning to inhibit motor movement (i.e., “Nogo signal”). Thus, increased phasic dopamine is responsible for learning from positive outcomes whereas decreased phasic dopamine is responsible for learning to avoid negative outcomes (Frank et al., 2004, 2005). In this example, it is clear that the striatum can contribute to both approach and avoidance learning. However, as a matter of illustrating the link between approach and avoidance motivation and BI, we largely focus on neural regions typically thought to independently mediate approach and avoidance motivation.
3. Avoidance Motivation
In our proposed model, childhood BI is in part the result of increased activation of the avoidance motivation system, particularly in contexts in which threat is not clearly defined. Avoidance motivation, likely the most extensively examined motivational system (LeDoux, 1995), is typically activated when threats are clearly defined (R. J. Blanchard & Blanchard, 1969; Davis, 1992, 1998; LeDoux, 1998). However, avoidance motivation can also be activated by contextual factors associated with threat (Davis et al., 2009).
Heightened avoidance motivation has been long theorized to play a central role in fear and anxiety (D. C. Blanchard & Blanchard, 1972; R. J. Blanchard et al., 1993; Fanselow, 1994), as well as in childhood BI (Coll et al., 1984; Kagan et al., 1987). Two neural regions appear to mediate avoidance motivation. First, a large body of work links the amygdala, a strongly conserved limbic structure, to immediate threat detection and motivational behaviors that remove the organism from harm (Davis, 1992; LeDoux, 1995). The second structure, the BNST, a limbic region adjacent to the amygdala, has been increasingly implicated in the pathogenesis of anxiety (Rauch, Shin, & Wright, 2003). The BNST has extensive connections with the amygdala and the striatum (Avery et al., 2014), suggesting the BNST plays a critical role in mediating motivational responding to the environment. The BNST has been closely linked to immediate threat detection (Shackman & Fox, 2016) and to sustained defensive motion when threat is unpredictable and nonspecific (Avery et al., 2016; Avery et al., 2014; Davis, 1998; Davis et al., 2009; Hammack, Richey, Watkins, & Maier, 2004; Lebow & Chen, 2016; Sullivan et al., 2004; Walker, Toufexis, & Davis, 2003). Here, we review work that has examined avoidance motivation in childhood BI, with a particular focus on activation of both amygdala and BNST, and how contextual factors may influence activation of the avoidance system.
The majority of research that has examined avoidance motivation in childhood BI has focused on the startle response, which is a whole-body movement elicited by the presentation of a surprising (and often abrasive) stimulus and is characterized by forward thrusting of the head followed by a descending flexor wave extending through the trunk and knees (Landis & Hunt, 1939). The startle response appears to be mediated by both the amygdala (LeDoux, 1995, 1998) and the BNST (Davis, 1998; Davis et al., 2009). Startle studies in childhood BI have generally found a heightened startle response during the presentation of threat cues (Barker et al., 2014; Barker, Reeb-Sutherland, et al., 2015; Schmidt & Fox, 1998), and heightened startle responding among behaviorally inhibited children increases the risk for anxiety (Barker, Reeb-Sutherland, et al., 2015; Reeb-Sutherland et al., 2009). Enhanced startle potentiation to threat has also been found to relate to anxiety (Davis et al., 2009; Grillon, 2002). Taken together, the results from startle studies suggest heightened avoidance motivation in childhood BI, particularly in the presence of potential threat.
Neuroimaging research has increasingly explored the relation between avoidance motivation and BI. Some studies have shown that compared with less-inhibited adolescents, those with a history of BI exhibit increased amygdala activity during subject ratings of fear (Perez-Edgar et al., 2007). Increased activation of the dorsolateral PFC, a region that is highly connected with the amygdala (Corbetta & Shulman, 2002), has also been observed during threat processing (Fu, Taber-Thomas, & Pèrez-Edgar, 2017). A number of studies have examined avoidance motivation in BI when processing novelty, since novelty is a known contextual factor that increases activation of motivational systems (Gray & McNaughton, 2003; Kagan et al., 1984; Kagan, Reznick, Snidman, Gibbons, & Johnson, 1988). It has been observed that, compared with adults with no history of BI, adults previously characterized by BI exhibited increased amygdala activity while processing novelty (Schwartz et al., 2003) and while processing unpredictable threat (Perez-Edgar et al., 2007). In addition, compared with adults with no history of BI, adults reporting early childhood BI display amygdala activity that does not habituate to novelty (Blackford et al., 2013), and the time course of amygdala activation (as measured during functional neuroimaging) peaks earlier to novelty at the trial level (Blackford, Avery, Shelton, & Zald, 2009).
In summary, the findings reviewed thus far suggest that childhood BI is characterized by an increased sensitivity of the avoidance motivational system. Thus, the evidence supports our proposed model shown in Figure 1 and our first hypothesis; that is, higher activation of avoidance motivation may result in greater likelihood of experiencing approach-avoidance conflict, resulting in inhibition. Also, it appears that BI is characterized by enhanced activation of the avoidance motivational system particularly in the presence of novelty, a known contextual factor that increases activation of motivational systems and ultimately leads to approach-avoidance conflict (Gray & McNaughton, 2003), supporting our second hypothesis that BI is characterized by an increased sensitivity to contextual factors, which lead to enhance conflict detection.
4. Approach Motivation
Approach-related motivation is characterized by goal-directed appetitive behaviors (Bijttebier, Beck, Claes, & Vandereycken, 2009; Corr, 2004; Gray, 1970, 1981). Heightened approach motivation plays a critical role in approach-avoidance conflict, the proposed cause of inhibition (Gray & McNaughton, 2003). As presented in Figure 1, our model suggests that along with increased sensitivity of the avoidance system, childhood BI is also related to increased sensitivity of the approach system. This theory at first glance may seem contradictory. However, using Gray’s theory that inhibition is the result of activation of both approach and avoidance motivation systems (Gray & McNaughton, 2003), we argue that childhood BI is similarly the result of increased activation of the approach system as well as the avoidance system.
Several regions of the brain have been identified as those that play a role in mediating approach motivation, including the striatum and cingulate (Corr, 2004; Gray & McNaughton, 2003). Recent work in animal neuroscience has further delineated the role of reward in approach motivation (Berridge, 2004; Berridge & Robinson, 2003; Berridge et al., 2009), which is primarily delivered by dopaminergic pathways arising from the striatum (Koepp et al., 1998; O’Doherty et al., 2004; Schultz, 2007; Schultz, Apicella, Scarnati, & Ljungberg, 1992; Schultz, Dayan, & Montague, 1997). In addition, neuroimaging and physiological studies have found that individual differences in approach motivation are related to varying degrees of activation of regions critical for reward processing (Bartussek, Diedrich, Naumann, & Collet, 1993; De Pascalis, Fiore, & Sparita, 1996; Simon et al., 2010).
Approach motivation in relation to childhood BI has also been increasingly studied. In general, individuals characterized with BI exhibit heightened activation of the approach motivational system (see Helfinstein, Fox, & Pine, 2012, for a more extensive review of this theory). While investigating approach motivation in relation to BI, Hardin and colleagues (2006) examined behavioral approach and avoidance motivation among shy adults, who display many phenotypic similarities to BI (Clauss & Blackford, 2012). The authors used the Monetary Incentive Delay Task (MID; Knutson, Adams, Fong, & Hommer, 2001; Schultz et al., 1992), during which participants are asked to respond to a target stimulus as fast as possible after cues that indicate the possibility of receiving a monetary reward (i.e., reward condition) or incurring a monetary loss (i.e., punishment condition). Across all participants, reaction times to reward and punishment trials were faster than to no-incentive trials, indicating that participants were sensitive to both reward and punishment. Critically, shy adults exhibited faster response times to both the reward and punishment condition compared with the non-shy group, indicating that shy adults are characterized by an increased activation of both approach and avoidance motivational systems. This initially counterintuitive finding further spurred the exploration of a relationship between BI and heightened approach motivation.
On the basis of these initial findings, Guyer and colleagues (2006) investigated reward sensitivity by using the MID task during functional magnetic response imaging in young adolescents (ages 10 −15 years) previously characterized as behaviorally inhibited. The authors observed that behaviorally inhibited adolescents exhibited increased activity within the striatum to both large potential gains and large potential losses. Similarly, Bar-Haim and colleagues (2009) examined striatal activations during a reward contingency task and found that behaviorally inhibited adolescents (ages 14 −18 years) exhibited greater striatal activation to stimuli in which participants had to make a choice to receive a reward. The link between BI and striatal activation appears to be strongest among adolescents with BI who also have genetic polymorphisms that promote dopamine production (Perez-Edgar et al., 2013), a key neurotransmitter for reward (Schultz, 2007). BI also appears to be characterized by enhanced striatal responses during anticipation and reception of social rewards (i.e., social approval; Guyer et al., 2014), and BI is also related to enhanced striatal activation to faces (Jarcho et al., 2013; Jarcho et al., 2014). Such findings suggest that individuals characterized with early BI demonstrate increased approach sensitivity to contextual factors related to social motivation (Geen, 1991, 1995).
Taken together, these findings present emerging evidence that BI is characterized by increased approach motivation (Helfinstein et al., 2012), supporting our first hypothesis that BI is the result of increased baseline activity of the approach motivational system. Although somewhat unintuitive, these findings are in line with Gray’s theory of inhibition, in which inhibition is the result of coactivation of approach and avoidance motivational systems (Bijttebier et al., 2009; Corr, 2004; Gray & McNaughton, 2003). In addition, findings from Guyer and colleagues (2014) point to the importance of social contexts as an activator of the approach system, offering support for our second hypothesis that BI is characterized by increased sensitivity of contextual factors.
5. Approach-Avoidance Conflict
Approach-avoidance conflict is central to inhibition (Aupperle & Paulus, 2010; Corr, 2004; Fowles, 1988; Gray & McNaughton, 2003; Kagan et al., 1984; Kagan et al., 1987). It has been suggested that approach-avoidance conflict is the result of either enhancement of both approach and avoidance motivation or an imbalance of these systems (Aupperle & Paulus, 2010). In addition, such enhancement or imbalance of approach and avoidance motivation is theorized to result in inhibition (Aupperle & Paulus, 2010). Gray argued that approach-avoidance conflict activated the BIS, which includes a number of neural regions, including the cingulate cortex, amygdala, and the hippocampus (Gray & McNaughton, 2003). Similarly, in our model of BI (see Figure 1), we argue that increased activation of both approach and avoidance motivational systems results in greater approach-avoidance conflict, causing increased activation of conflict monitoring system. In the following section, we first review neurobiological systems activated during the processing of conflict. Second, we examine neural functioning of conflict-processing regions in BI, which we argue underlies inhibition (see Amodio, Master, Yee, & Taylor, 2008, for a similar argument).
Monitoring of ongoing behavior in relation to environmental demands is critical for adapting behavior to achieve task goals. Monitoring and detecting the presence of conflict is one mechanism by which organisms adapt to the environment. Some theories suggest that conflict monitoring is central to motivated, goal-directed behavior (Botvinick et al., 2001; Carter & van Veen, 2007). Conflict, as defined by theories of conflict monitoring and cognitive control, refers to the coactivation of competing motor programs that results in response competition (Botvinick, Nystrom, Fissell, Carter, & Cohen, 1999; Carter & van Veen, 2007; Yeung et al., 2004). Detection of such conflict is thought to then trigger the recruitment of additional control (Botvinick et al., 2001).
As described in Figure 1, the conflict monitoring system is largely mediated by the cingulate cortex, including the ACC and PCC, as well as the prefrontal cortex (see Figure 2; Agam et al., 2011; Botvinick et al., 2001; Braver et al., 2001; Carter et al., 1998). Influential theories on ACC function suggest that the ACC plays a critical role in detecting deviations of goal-directed behavior and interfacing with the PFC to carry out the execution of goal-directed behavior (Holroyd & Yeung, 2012). Evidence of the role of the ACC in conflict monitoring comes from neuroimaging studies finding increased ACC activation when processing high-conflict trials (Botvinick et al., 1999; Braver, Barch, Gray, Molfese, & Snyder, 2001), and ACC activation during conflict processing predicts a number of behavioral adjustments to adjust goal-directed behavior (Danielmeier & Ullsperger, 2011). In addition, a number of event-related potentials have been identified that are generated by the ACC during conflict processing (Yeung et al., 2004), which we will detail more later.
Although the term conflict as it relates to cognitive control was developed from a different psychological disciple from the term conflict as used by Gray and Kagan, we argue that the processing of conflict in these situations recruits similar neural regions and drives similar behavioral changes, and may in fact refer to the same construct (see Amodio et al., 2008, for a similar argument). For example, both competing motor responses (e.g. conflict between two potential responses) and inhibition of a single motor response (reflecting conflict over whether to respond at all) elicit increased ACC activity (Carter et al., 1998; Donkers & van Boxtel, 2004; Nieuwenhuis, Yeung, van den Wildenberg, & Ridderinkhof, 2003). In addition, tasks that elicit high approach-avoidance conflict (i.e., participants are asked to produce a motor behavior in response to fearful or undesirable stimuli; Aupperle, Sullivan, Melrose, Paulus, & Stein, 2011; Rinck & Becker, 2007) yield increased activation of the ACC (Aupperle et al., 2015).
Along these lines, a more general view of the conflict monitoring system suggests that the system is sensitive to surprising events or to an event that is better/worse than expected (Alexander & Brown, 2011; Wessel & Aron, 2017). For example, Wessel and Aron (2017) suggest that the conflict monitoring system detects surprising and unexpected events, which leads to an inhibition of motor behavior. Consistent with this notion, we have recently demonstrated that healthy adults can demonstrate attentional and behavioral dysruptions following error commision (Buzzell, Beatty, Paquette, Roberts, & McDonald, 2017). However, Wessel (2018) suggests that despite such apparent disruptions following errors, such a neurobehavioral phenomenon is typically adaptive overall, as it allows the individual to stop and attend to the unexpected stimulus or behavior before moving on. Building on such ideas, we argue that overactivation of this typically adaptive response may at least in part underlay the BI temperament (for a similar argument see: Buzzell, Troller-Renfree, et al., 2017).
According to Gray and Kagan (Gray & McNaughton, 2003; Kagan et al., 1984), motivational conflict is central to inhibition. Thus, one would expect that BI and anxiety would be characterized by increased activation of the conflict monitoring system, as indexed by increased neural activation of systems associated with conflict monitoring, such as the cingulate cortex (Botvinick et al., 2001; Carter & van Veen, 2007). Indeed, this appears to be the case (Amodio et al., 2008; Buzzell, Troller-Renfree, et al., 2017; Lahat, Lamm, et al., 2014; McDermott et al., 2009; Moser, Moran, Schroder, Donnellan, & Yeung, 2013). Here, we focus on two interrelated features of conflict monitoring: neural activation during stimulus and motor conflict (Botvinick et al., 2001; Carter & van Veen, 2007) and neural processing during error commission (Falkenstein, Hoormann, Christ, & Hohnsbein, 2000; Gehring, Goss, Coles, Meyer, & Donchin, 1993).
One electrophysiological component of conflict monitoring, known as the N2, is a neural component believed to be primarily generated in the ACC and surrounding regions during high-conflict situations (Folstein & Van Petten, 2008; Kopp, Rist, & Mattler, 2007). It has been suggested that the N2 detects the need for inhibiting incorrect motor responding during such high-conflict situations (Botvinick et al., 2001; Yeung et al., 2004). Studies of the relation between N2 magnitude and BI have found that children (7 −8 years of age) characterized by early BI exhibit larger N2 magnitudes during a motor inhibition task (i.e., Go/No-Go Task) compared with less-inhibited children (Lamm et al., 2014). Enhanced N2 activation among behaviorally inhibited children (7 −8 years of age) has also been observed during a stimulus conflict task (Lahat, Walker, et al., 2014). During a dot-probe task, behaviorally inhibited children (7 −8 years of age) exhibit larger N2 amplitude when avoiding threat (Thai, Taber-Thomas, & Perez-Edgar, 2016). Amodio and colleagues (2008) investigated N2 amplitude in adults characterized by inhibition, as measured by the BIS/BAS Scales (Carver & White, 1994), and found that higher BIS scores predicted larger N2 amplitude. Overall, findings suggest that individual differences in BI are related to enhanced activation of the conflict monitoring system, as measured by the N2 component. In addition, research by Thai and colleagues (2016) suggested a link between avoidance motivation and increased conflict monitoring, a finding that merits further exploration.
Recent studies have used neuroimaging methods to investigate the relation between motivational conflict and BI. Jarcho and colleagues (2013) used an emotional Stroop task to examine conflict monitoring and found that adults with a history of childhood BI exhibited enhanced dorsomedial PFC activity during conflict detection. Interestingly, adults with childhood BI were also characterized by greater striatum activity during conflict adaptation. Using an attention-emotion conflict task, Jarcho and colleagues (2014) found that adults characterized by early childhood BI exhibited greater neural activity in conflict monitoring regions, such as the cingulate cortex, and increased activity in reward regions, such as the striatum.
Error processing has been theorized to be a core component of the conflict monitoring system (Holroyd & Coles, 2002; Yeung et al., 2004) and is theorized to reflect motivational responding to the environment (Hajcak, 2012; Proudfit, Inzlicht, & Mennin, 2013). Increasing approach motivation (through the delivery of reward) and increasing avoidance motivation (through the delivery of punishment) influence activation of the error monitoring system (Hajcak & Foti, 2008; Hajcak, Moser, Yeung, & Simons, 2005). In addition, factors that influence social motivation also modulate error monitoring (Barker, Troller-Renfree, Pine, & Fox, 2015; Barker, Troller-Renfree, Bowman, Pine, & Fox, 2018; Buzzell, Troller-Renfree, et al., 2017), suggesting that error processing is particularly sensitive to contextual factors that influence motivation. Studies of the error monitoring system have generally found that BI and anxiety are related to overactive error processing (Buzzell, Troller-Renfree, et al., 2017; McDermott et al., 2009; Moser et al., 2013), suggesting possible commonalities between inhibition and error monitoring.
One well-studied psychophysiological measure of error processing is error-related negativity (ERN), which is an event-related potential observed following error commission (Falkenstein, Hohnsbein, Hoormann, & Blanke, 1991; Gehring et al., 1993) and is likely generated by the ACC (Agam et al., 2011; Buzzell, Richards, et al., 2017). A number of studies have investigated ERN in relation to BI. McDermott and colleagues (2009) found that adolescents (14–16 years of age) identified with BI in early childhood exhibited an enhanced ERN as compared with adolescents without a history of BI. In an independent sample, Lahat, Lamm and colleagues (2014) measured the ERN in middle childhood among children previously identified as behaviorally inhibited at age 2 and 3 years. In a follow-up study of the same sample during early adolescence, Buzzell, Troller-Renfree, and colleagues (2017) found that adolescents (12–14 years of age) characterized by early BI exhibited a larger ERN than did adolescents without a history of childhood BI, particularly while under social observation.
Taken together, the evidence from both neuroimaging and psychophysiological studies suggest that childhood BI is characterized by an overactivation of the conflict monitoring system, which, according to our model and to Gray’s model (Gray & McNaughton, 2003), is the result of approach-avoidance conflict. In addition, findings regarding the error monitoring system in BI strongly and similarly suggest that BI is characterized by an enhanced conflict monitoring system. Accordingly, in our proposed model (Figure 1), increased approach-avoidance conflict leads to inhibition of behavior (Gray & McNaughton, 2003; Wessel, 2018; Wessel & Aron, 2017). However, no research reviewed previously in this article determined how overactivation of motivational systems leads to approach-avoidance conflict among children characterized as behaviorally inhibited. That is, do contextual factors known to increase activation of the approach and avoidance motivation cause overactivation of the conflict monitoring system among children characterized by BI? We attempt to answer this question in the following section.
6. Why Is Childhood BI Specifically a Risk Factor for Social Anxiety?
The findings we have reviewed suggest several similarities between anxiety and childhood BI in the enhanced activation of avoidance and approach motivational systems, as well as overactivation of the conflict monitoring system. However, it is important to note that BI is specifically a risk factor for social anxiety as opposed to other anxiety disorders (Biederman et al., 2001; Chronis-Tuscano et al., 2009; Clauss & Blackford, 2012; Hirshfeld et al., 1992). It is not entirely clear why BI most closely resembles phenotypic expressions of social anxiety, however it has been theorized that BI results in social anxiety because unfamiliar contexts are primarily social in nature during school-age years (Asendorpf, 1990b, 1991). Indeed, a number of studies have found that childhood BI predicts inhibition during play with unfamiliar peers (Fox et al., 2001; Rubin et al., 2002).
We expand upon this theory and suggest that social contexts, particularly those with unfamiliar peers, are strong activators of both approach and avoidance motivational systems, as contexts with unfamiliar peers often contain a high degree of novelty, ambiguity, and unpredictability. Thus, BI and social anxiety share a commonality of approach-avoidance conflict during social interactions. Approach-avoidance conflict has also been suggested to contribute to the development of social anxiety disorder (SAD; Aupperle & Paulus, 2010; Gilbert, 2001; Kashdan, Elhai, & Breen, 2008; Neal & Edelmann, 2003; Turner, Beidel, & Townsley, 1990). Specifically, SAD is characterized as excessive anxiety elicited by unfamiliar social situations (American Psychiatric Association, 2013; Stein & Stein, 2008), particularly in situations in which social evaluation might take place (Leary, 1983; Schlenker & Leary, 1982). We are not the first to make this connection; theoretical models of social motivation have also suggested that social anxiety may reflect enhanced social motivation (Geen, 1991, 1995). In the following section, we briefly review recent research that has attempted to explore how social contexts appear to be critical to modulating approach-avoidance conflict as a starting point to understanding relations between childhood BI and social anxiety.
Recent work has examined how contexts that influence motivation modulate the error monitoring system among socially anxious individuals. Barker, Troller-Renfree, and colleagues (2015) examined the ERN in young adults characterized as low or high in social anxiety symptoms. Participants completed a flanker task across two different contexts. In the nonsocial condition, participants completed a flanker task while alone in a room (i.e., alone condition). In the social condition, participants completed the same task while being observed and evaluated by a peer (i.e., peer condition). The authors found no differences in the ERN in the alone condition between high and low socially anxious individuals. However, in the social condition, socially anxious individuals exhibited a larger ERN in the peer condition as compared with low socially anxious individuals. These findings suggest the conflict monitoring system among socially anxious adults is specifically sensitive to social context, which is known to increase motivation (Geen, 1991, 1995). However, it is unknown whether changes in ERN magnitude among socially anxious individuals is the result of increases in approach motivation, avoidance motivation, or both.
Because BI is specifically related to the development of social anxiety (Clauss & Blackford, 2012), it is possible that children and adolescents characterized by BI would similarly exhibit enhanced error monitoring in social contexts. To examine the ERN in social contexts in a developmental sample, Barker and colleagues (2018) developed a flanker task in which participants are led to believe that two peers are observing and evaluating their performance via webcam and a chatroom. Using this task, Buzzell, Troller-Renfree, and colleagues (2017) examined the ERN in social and nonsocial contexts among adolescents identified as high and low in BI in early childhood. They found that across the entire sample, the ERN was enhanced in social contexts. In addition, BI predicted an ERN that was larger in social contexts than in nonsocial contexts. Critically, slowing after errors, an index of orienting and motor inhibition following errors (Danielmeier & Ullsperger, 2011; Notebaert et al., 2009; Wessel, 2018), mediated the relation between childhood BI and adolescent self-report of social anxiety symptoms.
These findings offer initial evidence in support of our model, such that contextual factors that increase motivation cause increases in conflict monitoring and subsequent increases in orienting. Furthermore, greater ERN enhancements among adolescents with early BI further support our second hypothesis that BI may be characterized by early differences in overactivation of conflict detection during contexts with a high degree of novelty and ambiguity, which leads to inhibition.
Although we have focused on delineating the role of relatively independent neural structures associated with approach and avoidance, it is worth noting that other neural indicators of approach-avoidance have been extensively described in the literature. For example, frontal alpha, a dominant frequency measured by electroencephalogram (EEG) electrodes over the frontal lobe, has been extensively linked to motivational processes (Fowles, 1988; Harmon-Jones & Allen, 1997; Reznik & Allen, 2018). A wealth of research has found that greater relative left frontal asymmetry is related to approach motivation, whereas greater right frontal asymmetry is related to avoidance motivation (Coan & Allen, 2003; Davidson & Fox, 1982; Davidson, Ekman, Saron, Senulis, & Friesen, 1990; Sutton & Davidson, 1997), and that BI is characterized by right frontal asymmetry (Calkins, Fox, & Marshall, 1996; Fox et al., 2001). An interesting future line of research would be the exploration of how asymmetry relates to other neural measures of approach and avoidance motivation and approach-avoidance conflict.
7. Summary and Future Directions
The goal of this review was to examine the role of approach and avoidance motivation in relation to BI, a phenotype characterized by increased negative reactivity within unfamiliar environments, and to present a novel model of the role of approach and avoidance motivation in the development of inhibition. Specifically, we presented one possible mechanism of childhood
BI (see Figure 1) in which we argue that childhood BI can be characterized using the definition of inhibition developed by Gray (Gray & McNaughton, 2003), who maintains that inhibition is specifically the result of approach-avoidance conflict.
Using evidence from decades of research on the underlying neurobiology of childhood BI, we suggest that BI may be characterized by an increased activation of both approach and avoidance motivational systems, which appears to cause an increased likelihood of experiencing approach-avoidance motivational conflict. In addition, growing evidence suggests that BI is characterized by an increased sensitivity to contextual factors, particularly those with a high degree of novelty, ambiguity, and unpredictability. Similarly, social contexts, a factor known to increase both motivational systems (Geen, 1991, 1995), also appear to be a strong factor in increased activation of motivational systems associated with childhood BI, and may partially explain why BI is specifically a risk factor for social anxiety (Clauss & Blackford, 2012). Such motivational conflict elicits downstream activation of conflict monitoring systems that serve to inhibit behavior, leading to increased physiological arousal and onlooking behavior.
BI shares many neural features of anxiety, namely, increased activation of neural structures associated with avoidance motivation, such as the amygdala and BNST (Barker et al., 2014; Grillon, 2002; Schmidt & Fox, 1998). In addition, BI and anxiety both exhibit enhancement of conflict monitoring via enhanced ACC activation during conflict monitoring (Buzzell, Troller-Renfree, et al., 2017; Gehring, Himle, & Nisenson, 2000; Lahat, Lamm, et al., 2014; McDermott et al., 2009; Moser et al., 2013; Ursu, Stenger, Shear, Jones, & Carter, 2003). In line with these findings, we suggest that motivational factors, both internal (i.e., innate differences in motivational responding to the environment) and external (i.e., presence of unfamiliarly peers), can enhance and/or imbalance motivational processes, setting the stage for approach-avoidance conflict (Aupperle & Paulus, 2010), and subsequent inhibition.
Acknowledgments
This research was partially supported by the National Institutes of Health (grant No. HDR3717899, to N.A.F.). T. V. B. was partially supported by National Institute of Health Fellowship (grant No. 5T32HD007542). The authors declare no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agam Y, Hämäläinen MS, Lee AKC, Dyckman KA, Friedman JS, Isom M, … Manoach DS (2011). Multimodal neuroimaging dissociates hemodynamic and electrophysiological correlates of error processing. Proceedings of the National Academy of Sciences, 108(42), 17556–17561. 10.1073/pnas.1103475108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander WH, & Brown JW (2011). Medial prefrontal cortex as an action-outcome predictor. Nature Neuroscience, 14(10), 1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, Master SL, Yee CM, & Taylor SE (2008). Neurocognitive components of the behavioral inhibition and activation systems: Implications for theories of self-regulation. Psychophysiology, 45(1), 11–19. 10.1111/j.1469-8986.2007.00609.x [DOI] [PubMed] [Google Scholar]
- Asendorpf JB (1990a). Beyond social withdrawal: Shyness, unsociability, and peer avoidance. Human Development, 33(4–5), 250–259. [Google Scholar]
- Asendorpf JB (1990b). Development of inhibition during childhood: Evidence for situational specificity and a two-factor model. Developmental Psychology, 26(5), 721. [Google Scholar]
- Asendorpf JB (1991). Development of Inhibited Children’s Coping with Unfamiliarity. Child Development, 62(6), 1460–1474. 10.1111/j.1467-8624.1991.tb01618.x [DOI] [PubMed] [Google Scholar]
- Association, A. P. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub. [DOI] [PubMed] [Google Scholar]
- Aupperle RL, Melrose AJ, Francisco A, Paulus MP, & Stein MB (2015). Neural substrates of approach-avoidance conflict decision-making. Human Brain Mapping, 36(2), 449–462. 10.1002/hbm.22639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle RL, & Paulus MP (2010). Neural systems underlying approach and avoidance in anxiety disorders. Dialogues in Clinical Neuroscience, 12(4), 517–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle RL, Sullivan S, Melrose AJ, Paulus MP, & Stein MB (2011). A reverse translational approach to quantify approach-avoidance conflict in humans. Behavioural Brain Research, 225(2), 455–463. 10.1016/j.bbr.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, & Blackford JU (2016). The Human BNST: Functional Role in Anxiety and Addiction. Neuropsychopharmacology, 41(1), 126–141. 10.1038/npp.2015.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery Suzanne N., Clauss JA, Winder DG, Woodward N, Heckers S, & Blackford JU (2014). BNST neurocircuitry in humans. NeuroImage, 91, 311–323. 10.1016/j.neuroimage.2014.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, & Hikosaka O (2007). The role of the dorsal striatum in reward and decision-making. Journal of Neuroscience, 27(31), 8161–8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Fox NA, Benson B, Guyer AE, Williams A, Nelson EE, … Ernst M (2009). Neural correlates of reward processing in adolescents with a history of inhibited temperament. Psychological Science, 20(8), 1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker TV, Reeb-Sutherland BC, & Fox NA (2014). Individual differences in fear potentiated startle in behaviorally inhibited children. Developmental Psychobiology, 56(1), 133–141. 10.1002/dev.21096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker TV, Reeb-Sutherland B, Degnan KA, Walker OL, Chronis-Tuscano A, Henderson HA, … Fox NA (2015). Contextual startle responses moderate the relation between behavioral inhibition and anxiety in middle childhood. Psychophysiology, 52(11), 1544–1549. 10.1111/psyp.12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker TV, Troller-Renfree S, Pine DS, & Fox NA (2015). Individual differences in social anxiety affect the salience of errors in social contexts. Cognitive, Affective, & Behavioral Neuroscience, 1–13. 10.3758/s13415-015-0360-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker TV, Troller-Renfree SV, Bowman LC, Pine DS, & Fox NA (2018). Social influences of error monitoring in adolescent girls. Psychophysiology, e13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartussek D, Diedrich O, Naumann E, & Collet W (1993). Introversion-extraversion and event-related potential (ERP): A test of JA Gray’s theory. Personality and Individual Differences, 14(4), 565–574. [Google Scholar]
- Beauchaine TP, & Zisner A (2017). Motivation, emotion regulation, and the latent structure of psychopathology: An integrative and convergent historical perspective. International Journal of Psychophysiology, 119, 108–118. 10.1016/jjjpsycho.2016.12.014 [DOI] [PubMed] [Google Scholar]
- Berridge KC (2004). Motivation concepts in behavioral neuroscience. Physiology & Behavior, 81(2), 179–209. 10.1016/j.physbeh.2004.02.004 [DOI] [PubMed] [Google Scholar]
- Berridge KC, & Robinson TE (2003). Parsing reward. Trends in Neurosciences, 26(9), 507–513. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, & Aldridge JW (2009). Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Current Opinion in Pharmacology, 9(1), 65–73. https://doi.org/10.1016Zj.coph.2008.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Hirshfeld-Becker DR, Rosenbaum JF, Herot C, Friedman D, Snidman N, … Faraone SV (2001). Further Evidence of Association Between Behavioral Inhibition and Social Children. American Journal of Psychiatry, 158(10), 1673–1679. 10.1176/appi.ajp.158.10.1673 [DOI] [PubMed] [Google Scholar]
- Bijttebier P, Beck I, Claes L, & Vandereycken W (2009). Gray’s Reinforcement Sensitivity Theory as a framework for research on personality-psychopathology associations. Clinical Psychology Review, 29(5), 421–430. 10.1016/j.cpr.2009.04.002 [DOI] [PubMed] [Google Scholar]
- Blackford Jennifer U., Avery SN, Shelton RC, & Zald DH (2009). Amygdala temporal dynamics: temperamental differences in the timing of amygdala response to familiar and novel faces. BMC Neuroscience, 10(1), 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford Jennifer Urbano, Allen AH, Cowan RL, & Avery SN (2013). Amygdala and hippocampus fail to habituate to faces in individuals with an inhibited temperament. Social Cognitive and Affective Neuroscience, 8(2), 143–150. 10.1093/scan/nsr078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, & Blanchard RJ (1972). Innate and conditioned reactions to threat in rats with amygdaloid lesions. Journal of Comparative and Physiological Psychology, 81(2), 281. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Pobbe R, & Blanchard RJ (2011). Risk assessment as an evolved threat detection and analysis process. Neuroscience & Biobehavioral Reviews, 35(4), 991–998. https://doi.org/10.1016Zj.neubiorev.2010.10.016 [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, & Blanchard DC (1969). Crouching as an index of fear. Journal of Comparative and Physiological Psychology, 67(3), 370. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Yudko EB, Rodgers RJ, & Blanchard DC (1993a). Defense system psychopharmacology: An ethological approach to the pharmacology of fear and anxiety. Behavioural Brain Research, 58(1), 155–165. 10.1016/0166-4328(93)90100-5 [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Yudko EB, Rodgers RJ, & Blanchard DC (1993b). Defense system psychopharmacology: An ethological approach to the pharmacology of fear and anxiety. Behavioural Brain Research, 58(1), 155–165. 10.1016/0166-4328(93)90100-5 [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, & Cohen JD (2001a). Conflict monitoring and cognitive control. Psychological Review, 108(3), 624–652. 10.1037/0033-295X.108.3.624 [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, & Cohen JD (2001b). Conflict monitoring and cognitive control. Psychological Review, 108(3), 624–652. 10.1037/0033-295X.108.3.624 [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, & Cohen JD (1999). Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature, 402(6758), 179–181. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, & Snyder A (2001). Anterior Cingulate Cortex and Response Conflict: Effects of Frequency, Inhibition and Errors. Cerebral Cortex, 11(9), 825–836. 10.1093/cercor/1L9.825 [DOI] [PubMed] [Google Scholar]
- Buzzell GA, Beatty PJ, Paquette NA, Roberts DM, & McDonald CG (2017). Error-Induced Blindness: Error Detection Leads to Impaired Sensory Processing and Lower Accuracy at Short Response-Stimulus Intervals. Journal of Neuroscience, 37(11), 2895–2903. 10.1523/JNEUROSCI.1202-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzell GA, Richards JE, White LK, Barker TV, Pine DS, & Fox NA (2017). Development of the error-monitoring system from ages 9–35: Unique insight provided by MRI-constrained source localization of EEG. NeuroImage, 157(Supplement C), 13–26. 10.1016/j.neuroimage.2017.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzell GA, Troller-Renfree SV, Barker TV, Bowman LC, Chronis-Tuscano A, Henderson HA, … Fox NA (2017a). A Neurobehavioral Mechanism Linking Behaviorally Inhibited Temperament and Later Adolescent Social Anxiety. Journal of the American Academy of Child & Adolescent Psychiatry. 10.1016/jjaac.2017.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzell GA, Troller-Renfree SV, Barker TV, Bowman LC, Chronis-Tuscano A, Henderson HA, … Fox NA (2017b). A Neurobehavioral Mechanism Linking Behaviorally Inhibited Temperament and Later Adolescent Social Anxiety. Journal of the American Academy of Child & Adolescent Psychiatry, 56(12), 1097–1105. 10.1016/jjaac.2017.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, Fox NA, & Marshall TR (1996). Behavioral and Physiological Antecedents of Inhibited and Uninhibited Behavior. Child Development, 67(2), 523–540. 10.1111/jM467-8624.1996.tb01749.x [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, & Cohen JD (1998). Anterior Cingulate Cortex, Error Detection, and the Online Monitoring of Performance. Science, 280(5364), 747–749. 10.1126/science.280.5364.747 [DOI] [PubMed] [Google Scholar]
- Carter CS, & Veen V van. (2007). Anterior cingulate cortex and conflict detection: An update of theory and data. Cognitive, Affective, & Behavioral Neuroscience, 7(4), 367–379. 10.3758/CABN.7A367 [DOI] [PubMed] [Google Scholar]
- Carver CS, & White TL (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology, 67(2), 319–333. 10.1037/0022-3514.67.2.319 [DOI] [Google Scholar]
- Cattell RB (1957). Personality and motivation structure and measurement.
- Chronis-Tuscano A, Degnan KA, Pine DS, Perez-Edgar K, Henderson HA, Diaz Y, … Fox NA (2009). Stable Early Maternal Report of Behavioral Inhibition Predicts Lifetime Social Anxiety Disorder in Adolescence. Journal of the American Academy of Child & Adolescent Psychiatry, 48(9), 928–935. 10.1097/CHI.0b013e3181ae09df [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LA, & Watson D (1991). Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology, 100(3), 316–336. 10.1037/0021-843X.1003.316 [DOI] [PubMed] [Google Scholar]
- Clauss JA, & Blackford JU (2012). Behavioral Inhibition and Risk for Developing Social Anxiety Disorder: A Meta-Analytic Study. Journal of the American Academy of Child & Adolescent Psychiatry, 51(10), 1066–1075.e1. 10.1016/jjaac.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA, Seay AL, Vanderklok RM, Avery S, Cao A, Cowan RL, … Blackford JU (2014). Structural and functional bases of inhibited temperament. Social Cognitive and Affective Neuroscience. 10.1093/scan/nsu019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA, & Allen JJ (2003). Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology, 40(1), 106–114. [DOI] [PubMed] [Google Scholar]
- Coll CG, Kagan J, & Reznick JS (1984). Behavioral inhibition in young children. Child Development, 1005–1019. [PubMed] [Google Scholar]
- Coplan RJ, Rubin KH, Fox NA, Calkins SD, & Stewart SL (1994). Being Alone, Playing Alone, and Acting Alone: Distinguishing among Reticence and Passive and Active Solitude in Young Children. Child Development, 65(1), 129–137. 10.1111/j.1467-8624.1994.tb00739.x [DOI] [PubMed] [Google Scholar]
- Corbetta M, & Shulman GL (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3(3), 201. [DOI] [PubMed] [Google Scholar]
- Corr PJ (2004). Reinforcement sensitivity theory and personality. Neuroscience & Biobehavioral Reviews, 28(3), 317–332. [DOI] [PubMed] [Google Scholar]
- Danielmeier C, & Ullsperger M (2011). Post-Error Adjustments. Frontiers in Psychology, 2 10.3389/fpsyg.2011.00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, & Fox NA (1982). Asymmetrical brain activity discriminates between positive and negative affective stimuli in human infants. Science, 218(4578), 1235–1237. 10.1126/science.7146906 [DOI] [PubMed] [Google Scholar]
- Davidson Richard J., Ekman P, Saron CD, Senulis JA, & Friesen WV (1990). Approach-withdrawal and cerebral asymmetry: Emotional expression and brain physiology: I. Journal of Personality and Social Psychology, 58(2), 330. [PubMed] [Google Scholar]
- Davis M (1992). The role of the amygdala in fear and anxiety. Annual Review of Neuroscience, 15(1), 353–375. [DOI] [PubMed] [Google Scholar]
- Davis M (1998). Are different parts of the extended amygdala involved in fear versus anxiety? Biological Psychiatry, 44(12), 1239–1247. 10.1016/S0006-3223(98)00288-1 [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, & Grillon C (2009). Phasic vs Sustained Fear in Rats and Humans: Role of the Extended Amygdala in Fear vs Anxiety. Neuropsychopharmacology, 35(1), 105–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pascalis V, Fiore AD, & Sparita A (1996). Personality, event-related potential (ERP) and heart rate (HR): An investigation of Gray’s theory. Personality and Individual Differences, 20(6), 733–746. [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, & Fiez JA (2000). Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology, 84(6), 3072–3077. [DOI] [PubMed] [Google Scholar]
- Digman JM (1990). Personality structure: Emergence of the five-factor model. Annual Review of Psychology, 41(1), 417–440. [Google Scholar]
- Donkers FCL, & van Boxtel GJM (2004). The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain and Cognition, 56(2), 165–176. https://doi.org/10.1016Zj.bandc.2004.04.005 [DOI] [PubMed] [Google Scholar]
- Eysenck HJ (1963). Biological basis of personality. Nature, 199(4898), 1031–1034. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, & Blanke L (1991). Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology, 78(6), 447–455. 10.1016/0013-4694(91)90062-9 [DOI] [PubMed] [Google Scholar]
- Falkenstein Michael, Hoormann J, Christ S, & Hohnsbein J (2000). ERP components on reaction errors and their functional significance: a tutorial. Biological Psychology, 51(2–3), 87–107. 10.1016/S0301-0511(99)00031-9 [DOI] [PubMed] [Google Scholar]
- Fanselow MS (1994). Neural organization of the defensive behavior system responsible for fear. Psychonomic Bulletin & Review, 1(4), 429–438. [DOI] [PubMed] [Google Scholar]
- Folstein JR, & Van Petten C (2008). Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology, 45(1), 152–170. 10.1111/j.1469-8986.2007.00602.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowles DC (1988). Psychophysiology and Psychopathology: A Motivational Approach. Psychophysiology, 25(4), 373–391. 10.1111/j.1469-8986.1988.tb01873.x [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, & Schmidt LA (2001). Continuity and Discontinuity of Behavioral Inhibition and Exuberance: Psychophysiological and Behavioral Influences across the First Four Years of Life. Child Development, 72(1), 1–21. 10.1111/1467-8624.00262 [DOI] [PubMed] [Google Scholar]
- Frank M (2005). Dynamic Dopamine Modulation in the Basal Ganglia: A Neurocomputational Account of Cognitive Deficits in Medicated and Nonmedicated Parkinsonism. Journal of Cognitive Neuroscience, 17(1), 51–72. 10.1162/0898929052880093 [DOI] [PubMed] [Google Scholar]
- Frank MJ, Seeberger LC, & O’Reilly RC (2004). By Carrot or by Stick: Cognitive Reinforcement Learning in Parkinsonism. Science, 306(5703), 1940–1943. 10.1126/science.1102941 [DOI] [PubMed] [Google Scholar]
- Fu X, Taber-Thomas BC, & Perez-Edgar K (2017). Frontolimbic functioning during threat-related attention: Relations to early behavioral inhibition and anxiety in children. Biological Psychology, 122, 98–109. 10.1016/j.biopsycho.2015.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geen RG (1991). Social Motivation. Annual Review of Psychology, 42(1), 377–399. 10.1146/annurev.ps.42.020191.002113 [DOI] [PubMed] [Google Scholar]
- Geen RG (1995). Human motivation: A social psychological approach. Thomson Brooks/Cole Publishing Co. [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, & Donchin E (1993). A Neural System for Error Detection and Compensation. Psychological Science, 4(6), 385–390. 10.1111/j.1467-9280.1993.tb00586.x [DOI] [Google Scholar]
- Gehring WJ, Himle J, & Nisenson LG (2000). Action-Monitoring Dysfunction in Obsessive- Compulsive Disorder. Psychological Science, 11(1), 1–6. 10.1111/1467-9280.00206 [DOI] [PubMed] [Google Scholar]
- Gilbert P (2001). EVOLUTION AND SOCIAL ANXIETY: The Role of Attraction, Social Competition, and Social Hierarchies. Psychiatric Clinics, 24(4), 723–751. 10.1016/S0193-953X(05)70260-4 [DOI] [PubMed] [Google Scholar]
- Goldberg LR (1993). The structure of phenotypic personality traits. American Psychologist, 48(1), 26. [DOI] [PubMed] [Google Scholar]
- Gorka SM, Nelson BD, Phan KL, & Shankman SA (2016). Intolerance of uncertainty and insula activation during uncertain reward. Cognitive, Affective, & Behavioral Neuroscience, 16(5), 929–939. 10.3758/s13415-016-0443-2 [DOI] [PubMed] [Google Scholar]
- Grant EC, & Mackintosh JH (1963). A comparison of the social postures of some common laboratory rodents. Behaviour, 21(3), 246–259. [Google Scholar]
- Gray JA (1981). A Critique of Eysenck’s Theory of Personality In Eysenck PHJ (Ed.), A Model for Personality (pp. 246–276). Springer; Berlin Heidelberg: Retrieved from http://link.springer.com.proxy-um.researchport.umd.edu/chapter/10.1007/978-3-642-67783-0_8 [Google Scholar]
- Gray Jeffrey A. (1970). The psychophysiological basis of introversion-extraversion. Behaviour Research and Therapy, 8(3), 249–266. [DOI] [PubMed] [Google Scholar]
- Gray Jeffrey A. (1982). On mapping anxiety. Behavioral and Brain Sciences, 5(3), 506–534. 10.1017/S0140525X00013297 [DOI] [Google Scholar]
- Gray JA, & McNaughton N (2003). The neuropsychology of anxiety: An enquiry into the function of the septo-hippocampal system. Oxford university press. [Google Scholar]
- Grillon C (2008). Models and mechanisms of anxiety: evidence from startle studies. Psychopharmacology, 199(3), 421–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon Christian. (2002). Startle reactivity and anxiety disorders: aversive conditioning, context, and neurobiology. Biological Psychiatry, 52(10), 958–975. 10.1016/S0006-3223(02)01665-7 [DOI] [PubMed] [Google Scholar]
- Guyer AE, Benson B, Choate VR, Bar-Haim Y, Perez-Edgar K, Jarcho JM, … Nelson EE (2014). Lasting associations between early-childhood temperament and late-adolescent reward-circuitry response to peer feedback. Development and Psychopathology, 26(1), 229–243. 10.1017/S0954579413000941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, … Fox NA (2006). Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. Journal of Neuroscience, 26(24), 6399–6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G (2012). What We’ve Learned From Mistakes: Insights From Error-Related Brain Activity. Current Directions in Psychological Science, 21(2), 101–106. 10.1177/0963721412436809 [DOI] [Google Scholar]
- Hajcak G, & Foti D (2008). Errors are aversive: defensive motivation and the error-related negativity. Psychological Science, 19(2), 103–108. 10.1111/j.1467-9280.2008.02053.x [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Yeung N, & Simons RF (2005). On the ERN and the significance of errors. Psychophysiology, 42(2), 151–160. 10.1111/j.1469-8986.2005.00270.x [DOI] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Watkins LR, & Maier SF (2004). Chemical Lesion of the Bed Nucleus of the Stria Terminalis Blocks the Behavioral Consequences of Uncontrollable Stress. Behavioral Neuroscience, 118(2), 443–448. 10.1037/0735-7044.118.2.443 [DOI] [PubMed] [Google Scholar]
- Hardin MG, Perez-Edgar K, Guyer AE, Pine DS, Fox NA, & Ernst M (2006). Reward and punishment sensitivity in shy and non-shy adults: Relations between social and motivated behavior. Personality and Individual Differences, 40(4), 699–711. 10.1016/j.paid.2005.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E, & Allen JJB (1997). Behavioral activation sensitivity and resting frontal EEG asymmetry: Covariation of putative indicators related to risk for mood disorders. Journal of Abnormal Psychology, 106(1), 159–163. 10.1037/0021-843X.106.L159 [DOI] [PubMed] [Google Scholar]
- Helfinstein SM, Fox NA, & Pine DS (2012). Approach-withdrawal and the role of the striatum in the temperament of behavioral inhibition. Developmental Psychology, 48(3), 815. [DOI] [PubMed] [Google Scholar]
- Hirshfeld DR, ROSENBAUM JF, BIEDERMAN J, BOLDUC EA, FARAONE SV, SNIDMAN N, … KAGAN J (1992). Stable Behavioral Inhibition and Its Association with Anxiety Disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 31(1), 103–111. 10.1097/00004583-199201000-00016 [DOI] [PubMed] [Google Scholar]
- Holroyd CB, & Coles MGH (2002). The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review, 109(4), 679–709. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, & Yeung N (2012). Motivation of extended behaviors by anterior cingulate cortex. Trends in Cognitive Sciences, 16(2), 122–128. 10.1016/j.tics.2011.12.008 [DOI] [PubMed] [Google Scholar]
- Horvitz JC (2000). Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience, 96(4), 651–656. 10.1016/S0306-4522(00)00019-1 [DOI] [PubMed] [Google Scholar]
- Jackson F, Nelson BD, & Hajcak G (2016). The uncertainty of errors: Intolerance of uncertainty is associated with error-related brain activity. Biological Psychology, 113, 52–58. 10.1016/j.biopsycho.2015.11.007 [DOI] [PubMed] [Google Scholar]
- Jarcho JM, Fox NA, Pine DS, Etkin A, Leibenluft E, Shechner T, & Ernst M (2013). The neural correlates of emotion-based cognitive control in adults with early childhood behavioral inhibition. Biological Psychology, 92(2), 306–314. 10.1016/j.biopsycho.2012.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho JM, Fox NA, Pine DS, Leibenluft E, Shechner T, Degnan KA, … Ernst M (2014). Enduring influence of early temperament on neural mechanisms mediating attention-emotion conflict in adults. Depression and Anxiety, 31(1), 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John OP, & Srivastava S (1999). The Big Five trait taxonomy: History, measurement, and theoretical perspectives. Handbook of Personality: Theory and Research, 2(1999), 102–138. [Google Scholar]
- Kagan J, Reznick JS, & Snidman N (1988). Biological Bases of Childhood Shyness. Science, 240(4849), 167–171. 10.1126/science.3353713 [DOI] [PubMed] [Google Scholar]
- Kagan Jerome, Reznick JS, Clarke C, Snidman N, & Garcia-Coll C (1984). Behavioral inhibition to the unfamiliar. Child Development, 2212–2225. [Google Scholar]
- Kagan Jerome, Reznick JS, & Snidman N (1986). Temperamental inhibition in early childhood. The Study of Temperament: Changes, Continuities and Challenges, 53–67. [Google Scholar]
- Kagan Jerome, Reznick JS, & Snidman N (1987). The Physiology and Psychology of Behavioral Inhibition in Children. Child Development, 58(6), 1459 10.2307/1130685 [DOI] [PubMed] [Google Scholar]
- Kagan Jerome, Reznick JS, Snidman N, Gibbons J, & Johnson MO (1988). Childhood Derivatives of Inhibition and Lack of Inhibition to the Unfamiliar. Child Development, 59(6), 1580–1589. 10.2307/1130672 [DOI] [PubMed] [Google Scholar]
- Kagan Jerome, & Snidman N (1999). Early childhood predictors of adult anxiety disorders. Biological Psychiatry, 46(11), 1536–1541. 10.1016/S0006-3223(99)00137-7 [DOI] [PubMed] [Google Scholar]
- Kashdan TB, Elhai JD, & Breen WE (2008). Social anxiety and disinhibition: an analysis of curiosity and social rank appraisals, approach-avoidance conflicts, and disruptive risk-taking behavior. Journal of Anxiety Disorders, 22(6), 925–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, & Hommer D (2001). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience, 21(16), RC159–RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepp MJ, Gunn RN, Lawrence AD, Cunningham VJ, Dagher A, Jones T, … Grasby PM (1998). Evidence for striatal dopamine release during a video game. Nature, 393(6682), 266. [DOI] [PubMed] [Google Scholar]
- Kopp Bruno, Rist Fred, & Mattler Uwe. (2007). N200 in the flanker task as a neurobehavioral tool for investigating executive control. Psychophysiology, 33(3), 282–294. 10.1111/j.1469-8986.1996.tb00425.x [DOI] [PubMed] [Google Scholar]
- Lahat A, Lamm C, Chronis-Tuscano A, Pine DS, Henderson HA, & Fox NA (2014). Early Behavioral Inhibition and Increased Error Monitoring Predict Later Social Phobia Symptoms in Childhood. Journal of the American Academy of Child & Adolescent Psychiatry, 53(4), 447–455. 10.1016/jjaac.2013.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat A, Walker OL, Lamm C, Degnan KA, Henderson HA, & Fox NA (2014). Cognitive conflict links behavioural inhibition and social problem solving during social exclusion in childhood. Infant and Child Development, 23(3), 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Walker OL, Degnan KA, Henderson HA, Pine DS, McDermott JM, & Fox NA (2014). Cognitive control moderates early childhood temperament in predicting social behavior in 7-year-old children: an ERP study. Developmental Science, 17(5), 667–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis C, & Hunt W (1939). The startle pattern (Vol. x). Oxford, England: Farrar & Rinehart. [Google Scholar]
- Leary MR (1983). A brief version of the Fear of Negative Evaluation Scale. Personality and Social Psychology Bulletin, 9(3), 371–375. [Google Scholar]
- Lebow MA, & Chen A (2016). Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Molecular Psychiatry, 21(4), 450–463. 10.1038/mp.2016.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J (1998). Fear and the brain: where have we been, and where are we going? Biological Psychiatry, 44(12), 1229–1238. 10.1016/S0006-3223(98)00282-0 [DOI] [PubMed] [Google Scholar]
- LeDoux JE (1995). Emotion: Clues from the brain. Annual Review of Psychology, 46(1), 209–235. [DOI] [PubMed] [Google Scholar]
- LeDoux JE (2014). Coming to terms with fear. Proceedings of the National Academy of Sciences, 111(8), 2871–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh NJ (1974). The psychology of animal learning. Academic Press. [Google Scholar]
- Marshall PJ, Reeb BC, & Fox NA (2009). Electrophysiological responses to auditory novelty in temperamentally different 9-month-old infants. Developmental Science, 12(4), 568–582. 10.1111/j.1467-7687.2008.00808.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, & Fox NA (2009a). A History of Childhood Behavioral Inhibition and Enhanced Response Monitoring in Adolescence Are Linked to Clinical Anxiety. Biological Psychiatry, 65(5), 445–448. https://doi.org/10.1016Z.biopsych.2008.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, & Fox NA (2009b). A History of Childhood Behavioral Inhibition and Enhanced Response Monitoring in Adolescence Are Linked to Clinical Anxiety. Biological Psychiatry, 65(5), 445–448. https://doi.org/10.1016Zj.biopsych.2008.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan BE (2006). Behavioral inhibition: A neurobiological perspective. Current Psychiatry Reports, 8(4), 270–278. 10.1007/s11920-006-0062-7 [DOI] [PubMed] [Google Scholar]
- Moser JS, Moran TP, Schroder HS, Donnellan MB, & Yeung N (2013). On the relationship between anxiety and error monitoring: a meta-analysis and conceptual framework. Frontiers in Human Neuroscience, 7 10.3389/fnhum.2013.00466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal JA, & Edelmann RJ (2003). The etiology of social phobia: Toward a developmental profile. Clinical Psychology Review, 23(6), 761–786. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, van den Wildenberg W, & Ridderinkhof KR (2003). Electrophysiological correlates of anterior cingulate function in a go/no-go task: Effects of response conflict and trial type frequency. Cognitive, Affective, & Behavioral Neuroscience, 3(1), 17–26. https://doi.Org/10.3758/CABN.3.1.17 [DOI] [PubMed] [Google Scholar]
- Notebaert W, Houtman F, Van Opstal F, Gevers W, Fias W, & Verguts T (2009). Post-error slowing: an orienting account. Cognition, 111(2), 275–279. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, & Dolan RJ (2004). Dissociable Roles of Ventral and Dorsal Striatum in Instrumental Conditioning. Science, 304(5669), 452–454. 10.1126/science.1094285 [DOI] [PubMed] [Google Scholar]
- Pèrez-Edgar K, Hardee JE, Guyer AE, Benson BE, Nelson EE, Gorodetsky E, … Ernst M (2013). DRD4 and striatal modulation of the link between childhood behavioral inhibition and adolescent anxiety. Social Cognitive and Affective Neuroscience, 9(4), 445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pèrez-Edgar K, Roberson-Nay R, Hardin MG, Poeth K, Guyer AE, Nelson EE, … Pine DS (2007). Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. Neuroimage, 35(4), 1538–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfit GH, Inzlicht M, & Mennin DS (2013). Anxiety and error monitoring: the importance of motivation and emotion. Frontiers in Human Neuroscience, 7 Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3792698/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeb-Sutherland BC, Helfinstein SM, Degnan KA, Perez-Edgar K, Henderson HA, Lissek S, … Fox NA (2009). Startle Response in Behaviorally Inhibited Adolescents With a Lifetime Occurrence of Anxiety Disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 48(6), 610–617. 10.1097/CHI.0b013e31819f70fb [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznik SJ, & Allen JJ (2018). Frontal asymmetry as a mediator and moderator of emotion: An updated review. Psychophysiology, 55(1), e12965. [DOI] [PubMed] [Google Scholar]
- Rinck M, & Becker ES (2007). Approach and avoidance in fear of spiders. Journal of Behavior Therapy and Experimental Psychiatry, 38(2), 105–120. 10.1016/j.jbtep.2006.10.001 [DOI] [PubMed] [Google Scholar]
- Rothbart Mary K. (1986). Longitudinal observation of infant temperament. Developmental Psychology, 22(3), 356–365. 10.1037/0012-1649.22.3.356 [DOI] [Google Scholar]
- Rothbart Mary K. (2007). Temperament, Development, and Personality. Current Directions in Psychological Science, 16(4), 207–212. 10.1111/j.1467-8721.2007.00505.x [DOI] [Google Scholar]
- Rothbart Mary Klevjord. (1981). Measurement of Temperament in Infancy. Child Development, 52(2), 569–578. 10.2307/1129176 [DOI] [Google Scholar]
- Rubin KH (2014). Social withdrawal, inhibition, and shyness in childhood. Psychology Press. [Google Scholar]
- Rubin KH, Bukowski WM, & Parker JG (1998). Peer interactions, relationships, and groups. Handbook of Child Psychology. Retrieved from http://onlinelibrary.wiley.com/doi/10.1002/9780470147658.chpsy0310/full [Google Scholar]
- Rubin KH, Burgess KB, & Hastings PD (2002). Stability and Social-Behavioral Consequences of Toddlers’ Inhibited Temperament and Parenting Behaviors. Child Development, 73(2), 483–495. 10.1111/1467-8624.00419 [DOI] [PubMed] [Google Scholar]
- Rubin KH, Cheah CSL, & Fox N (2001). Emotion Regulation, Parenting and Display of Social Reticence in Preschoolers. Early Education and Development, 12(1), 97–115. 10.1207/s15566935eed1201_6 [DOI] [Google Scholar]
- Rubin KH, Coplan RJ, & Bowker JC (2009). Social Withdrawal in Childhood. Annual Review of Psychology, 60(1), 141–171. 10.1146/annurev.psych.60.110707.163642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin KH, & Mills RS (1988). The many faces of social isolation in childhood. Journal of Consulting and Clinical Psychology, 56(6), 916. [DOI] [PubMed] [Google Scholar]
- Salamone JD, & Correa M (2012). The Mysterious Motivational Functions of Mesolimbic Dopamine. Neuron, 76(3), 470–485. 10.1016/j.neuron.2012.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenker BR, & Leary MR (1982). Social anxiety and self-presentation: A conceptualization model. Psychological Bulletin, 92(3), 641–669. 10.1037/0033-2909.92.3.641 [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Schulkin J, & Gold PW (1999). Behavioral and psychophysiological correlates of self-presentation in temperamentally shy children. Developmental Psychobiology, 35(2), 119–135. [PubMed] [Google Scholar]
- Schmidt Louis A, & Fox NA (1998). Fear-potentiated startle responses in temperamentally different human infants. Developmental Psychobiology, 32(2), 113–120. [DOI] [PubMed] [Google Scholar]
- Schultz W (2007). Multiple Dopamine Functions at Different Time Courses. Annual Review of Neuroscience, 30(1), 259–288. 10.1146/annurev.neuro.28.061604.135722 [DOI] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Scarnati E, & Ljungberg T (1992). Neuronal activity in monkey ventral striatum related to the expectation of reward. Journal of Neuroscience, 12(12), 4595–4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, & Montague PR (1997). A Neural Substrate of Prediction and Reward. Science, 275(5306), 1593–1599. 10.1126/science.275.5306.1593 [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Kunwar PS, Greve DN, Kagan J, Snidman NC, & Bloch RB (2012). A phenotype of early infancy predicts reactivity of the amygdala in male adults. Molecular Psychiatry, 17(10), 1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz Carl E., Wright CI, Shin LM, Kagan J, & Rauch SL (2003). Inhibited and Uninhibited Infants “Grown Up”: Adult Amygdalar Response to Novelty. Science, 300(5627), 1952–1953. 10.1126/science.1083703 [DOI] [PubMed] [Google Scholar]
- Shackman AJ, & Fox AS (2016). Contributions of the Central Extended Amygdala to Fear and Anxiety. Journal of Neuroscience, 36(31), 8050–8063. 10.1523/JNEUROSCI.0982-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Gorka SM, Nelson BD, Fitzgerald DA, Phan KL, & O’Daly O (2014). Anterior Insula Responds to Temporally Unpredictable Aversiveness: an fMRI Study. Neuroreport, 25(8), 596–600. 10.1097/WNR.0000000000000144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, & Liberzon I (2010). The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology, 35(1), 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JJ, Walther S, Fiebach CJ, Friederich H-C, Stippich C, Weisbrod M, & Kaiser S (2010). Neural reward processing is modulated by approach- and avoidance-related personality traits. NeuroImage, 49(2), 1868–1874. 10.1016/j.neuroimage.2009.09.016 [DOI] [PubMed] [Google Scholar]
- Smillie LD, Pickering AD, & Jackson CJ (2006). The new reinforcement sensitivity theory: Implications for personality measurement. Personality and Social Psychology Review, 10(4), 320–335. [DOI] [PubMed] [Google Scholar]
- Stein MB, & Stein DJ (2008). Social anxiety disorder. Lancet, 371(9618), 1115–1125. 10.1016/S0140-6736(08)60488-2 [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DEA, Johnson LR, Hou M, & Ledoux JE (2004). Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience, 128(1), 7–14. 10.1016/j.neuroscience.2004.06.015 [DOI] [PubMed] [Google Scholar]
- Sutton SK, & Davidson RJ (1997). Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychological Science, 8(3), 204–210. [Google Scholar]
- Thai N, Taber-Thomas BC, & Perez-Edgar KE (2016). Neural correlates of attention biases, behavioral inhibition, and social anxiety in children: An ERP study. Developmental Cognitive Neuroscience, 19, 200–210. 10.1016/j.dcn.2016.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, & Chess S (1977). Temperament and development. Brunner/Mazel. [Google Scholar]
- Turner SM, Beidel DC, & Townsley RM (1990). Social phobia: Relationship to shyness. Behaviour Research and Therapy, 28(6), 497–505. 10.1016/0005-7967(90)90136-7 [DOI] [PubMed] [Google Scholar]
- Ullsperger M, Danielmeier C, & Jocham G (2014). Neurophysiology of Performance Monitoring and Adaptive Behavior. Physiological Reviews, 94(1), 35–79. 10.1152/physrev.00041.2012 [DOI] [PubMed] [Google Scholar]
- Ursu S, Stenger VA, Shear MK, Jones MR, & Carter CS (2003). Overactive Action Monitoring in Obsessive-Compulsive Disorder Evidence From Functional Magnetic Resonance Imaging. Psychological Science, 14(4), 347–353. 10.1111/1467-9280.24411 [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, & Davis M (2003). Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. European Journal of Pharmacology, 463(1–3), 199–216. 10.1016/S0014-2999(03)01282-2 [DOI] [PubMed] [Google Scholar]
- Wessel JR (2018). An adaptive orienting theory of error processing. Psychophysiology, 55(3). 10.1111/psyp.13041 [DOI] [PubMed] [Google Scholar]
- Wessel JR, & Aron AR (2017). On the Globality of Motor Suppression: Unexpected Events and Their Influence on Behavior and Cognition. Neuron, 93(2), 259–280. 10.1016/j.neuron.2016.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR, Danielmeier C, Morton JB, & Ullsperger M (2012). Surprise and Error: Common Neuronal Architecture for the Processing of Errors and Novelty. The Journal of Neuroscience, 32(22), 7528–7537. 10.1523/JNEUROSCI.6352-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, & Cohen JD (2004). The Neural Basis of Error Detection: Conflict Monitoring and the Error-Related Negativity. Psychological Review, 111(4), 931–959. 10.1037/0033-295X.111A931 [DOI] [PubMed] [Google Scholar]