Fig. 1.
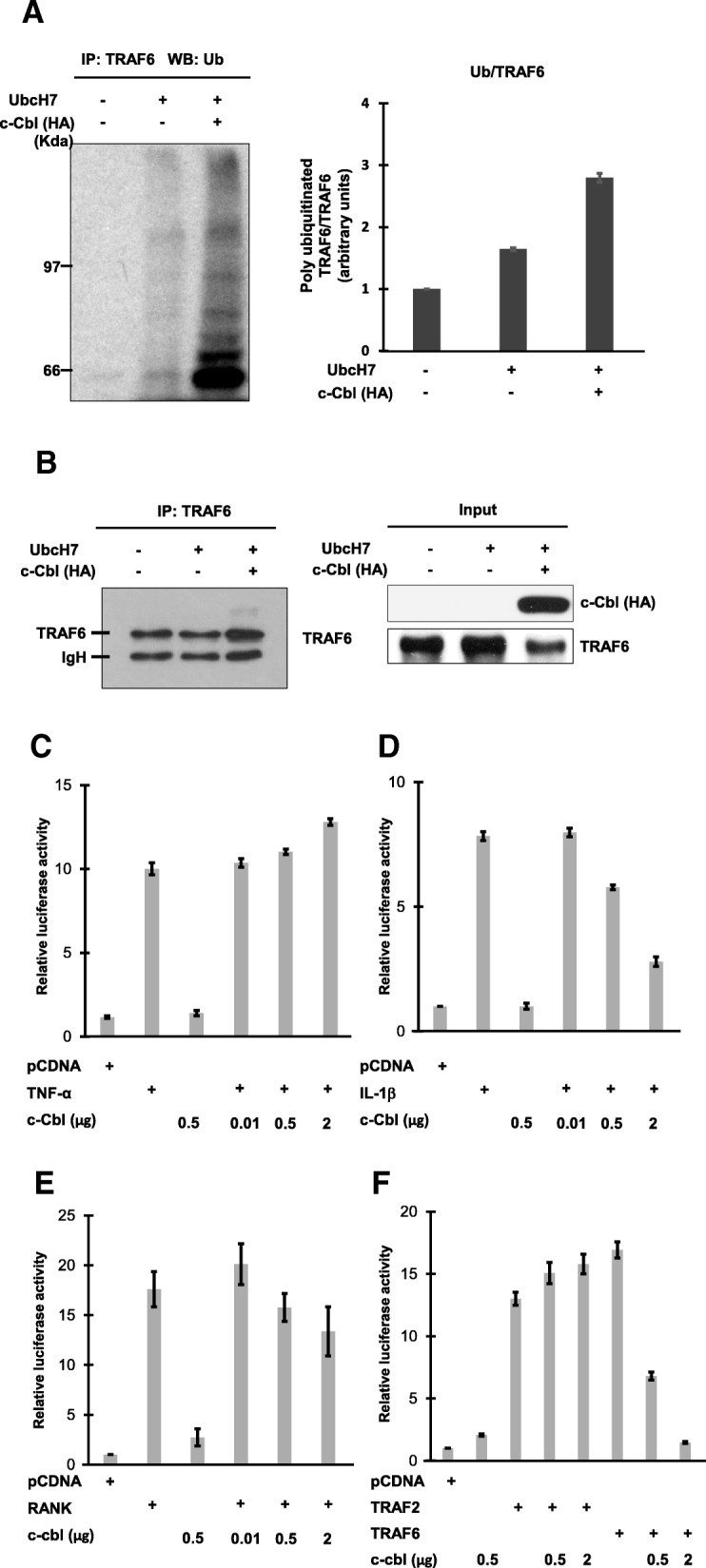
c-Cbl promotes TRAF6 ubiquitination and inhibits TRAF6 mediated NF-κB activation. a – In vitro ubiquitination assays with immunoprecipitated TRAF6 from 293 T cells, transfected with either c-Cbl or an empty vector, and UbcH7 as ubiquitin-conjugating (E2) enzymes. These proteins were added to ubiquitination reactions consisting of E1, ATP and Ub, as described in the Methods section. Ubiquitinated proteins were detected by immunoblotting with anti-ubiquitin antibody. Quantification of the TRAF6 ubiquitination level of Ub/TRAF6. b – Immunoprecipitation of TRAF6 followed by immunoblot analysis with anti-TRAF6 (left panel) is shown. Controls for the expressions of HA-cbl and TRAF6 are shown (right panel). c and d – c-Cbl inhibited IL-1β-induced NF-κB activation, but not TNF-α mediated NF-κB activation. 293 T cells were co-transfected with 50 ng of (κB)3-interferon–luciferase reporter plasmid, 25 ng of CMV-β-galactosidase plasmid, and 1 μg of pcDNA empty vector or HA-tagged pcDNA-c-Cbl, as indicated. 36 h after transfection, cells were treated with TNF-α (20 ng/ml) (c) or IL-1β (10 ng/ml) (d) for 6 h or left untreated. e – c-Cbl inhibited RANK mediated NF-κB activation. 293 T cells were co-transfected with reporter plasmids and either a plasmid encoding RANK alone or c-cbl plasmid (1 μg), as indicated. f – c-Cbl inhibited TRAF6, but not TRAF2, and mediated NF-kB activation. Expression plasmids encoding TRAF2 and TRAF6 were tested for NF-kB activity toward c-Cbl. The results shown in (c through f) represent the means ± SD of triplicate experiments
