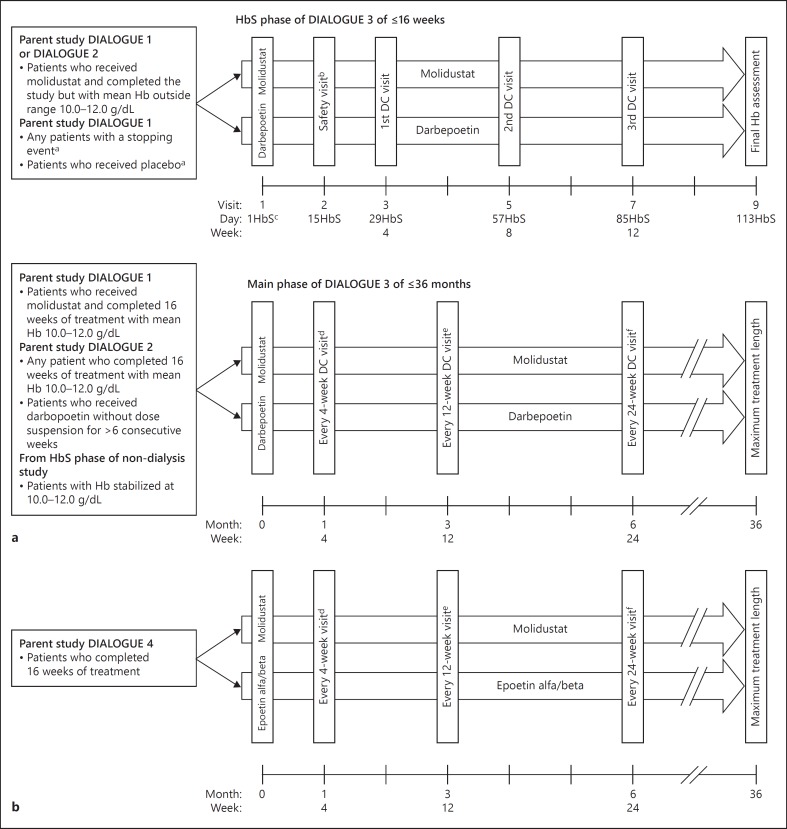
Fig. 1.
Study design of (a) DIALOGUE 3 and (b) DIALOGUE 5. a In the case of a patient who received placebo and completed DIALOGUE 1, eligibility for entry into the extension study could be reassessed ≤4 weeks after the DIALOGUE 1 end of treatment visit. b A safety visit was required only for patients in the darbepoetin arm and for patients in the molidustat arm who did not complete the day 15 safety visit or who had an Hb stopping event after day 15 in DIALOGUE 1. c ‘HbS' denotes the numbering of days in the HbS phase. d Every 4 (±1) weeks: Hb, dose decision. e Every 12 (±2) weeks: Hb, dose decision, and central laboratory tests. f Every 24 (±2) weeks: Hb, dose decision. DC, dose control; Hb, hemoglobin; HbS, Hb-stabilization.