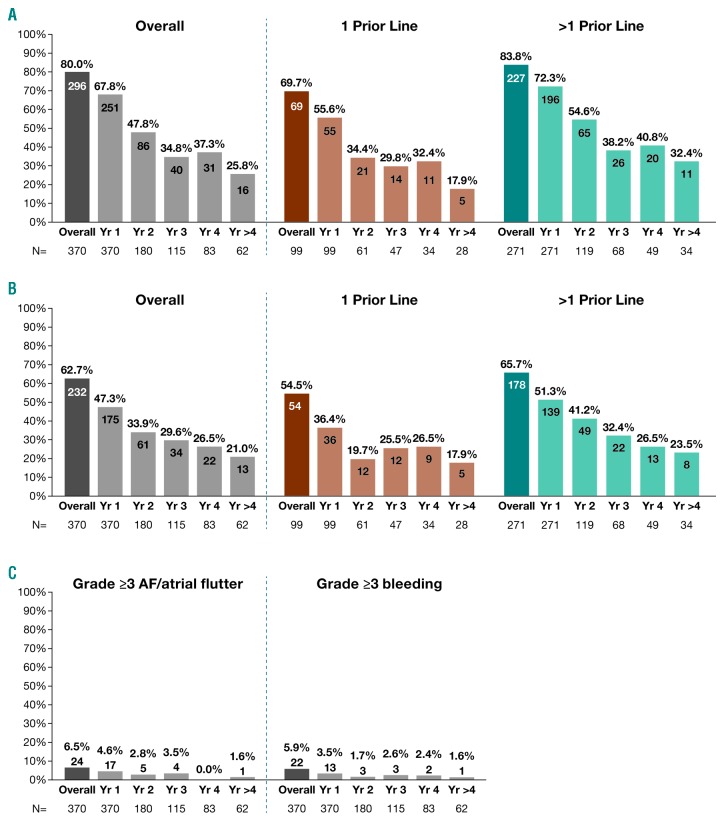
Figure 2.
Incidence of treatment-emergent adverse events (TEAEs) over time. (A) Grade ≥3 TEAEs generally decreased over time with lower rates associated with ibrutinib use in second versus later lines. (B) Any-grade serious TEAEs generally decreased over time with lower rates associated with ibrutinib use in second versus later lines. (C) Incidence of grade ≥3 AF and bleeding was highest in year 1 and decreased thereafter. Overall population is the full ibrutinib cohort enrolled in the original three studies (N=370), including those who continued on to the CAN3001 study. Number of patients with adverse events shown on bars. Recurrent events per patient were counted only once per year. If recurrent events occurred in a single patient in separate years, these were counted separately in each year (once only per year). N: number; AF: atrial fibrillation; Yr: year.