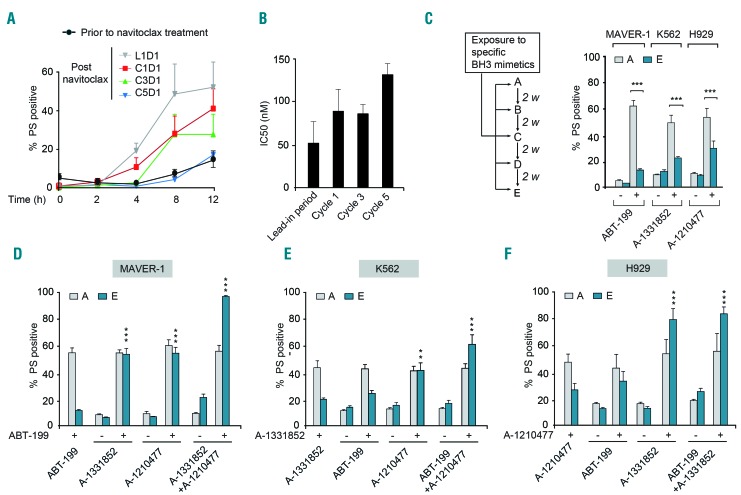
Figure 1.
Hematologic malignancies rapidly acquire resistance to BH3 mimetics. (A) Blood samples collected from patients with chronic lymphocytic leukemia (CLL) (n=5), either prior to the first in vivo dose of navitoclax or 4 h after dosing during different stages of treatment - day 1 of the initial lead-in-period (L1D1), day 1 of cycle 1 (C1D1), day 1 of cycle 3 (C3D1) or day 1 of cycle 5 (C5D1) - were incubated ex vivo and the extent of apoptosis in the CD19+ CLL cells was assessed at the indicated time points by measuring phosphatidylserine (PS) externalization. (B) CLL cells isolated from these patients at the beginning of each treatment cycle, as indicated in the figure, were exposed in vitro to increasing concentrations of ABT-263 and the extent of apoptosis was assessed: half maximal inhibitory concentration (IC50) values are shown. (C) Scheme for establishing resistance to specific BH3 mimetics in relevant hematologic cell lines, as explained in the Methods section. Sensitive [A] and resistant [E] cells of MAVER-1, K562 and H929 cell lines were exposed for 4 h to ABT-199 (10 nM), A-1331852 (10 nM) and A-1210477 (5 μM), respectively, and apoptosis was assessed. (D-F) Combinations with some but not all BH3 mimetics restored apoptotic sensitivity of resistant [E] MAVER-1, K562 and H929 cells exposed for 4 h to ABT-199 (10 nM), A-1331852 (10 nM) or A-1210477 (5 μM), respectively. ***P⩽0.001, **P⩽0.01. Error bars = mean ± standard error of mean (n=3).