Figure 3.
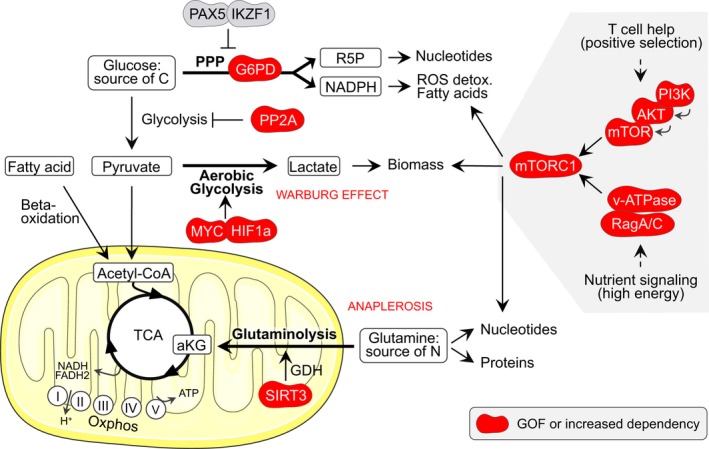
Metabolic dysregulation in GC‐derived B‐cell lymphoma. In the mitochondrion, the tricarboxylic acid (TCA) cycle produces reduced NADH and FADH2 that are used by complexes I to V of the electron transport chain (ETC) to generate ATP through oxidative phosphorylation (oxphos). The TCA can be fueled by fatty acid‐ or pyruvate‐derived acetyl‐CoA. Alternatively, glutamine can be used to generate alpha‐ketoglutarate (aKG). DLBCLs have developed dependency on SIRT3 to replenish the TCA cycle (also known as anaplerosis). SIRT3 stimulates glutaminolysis by activating the glutamine dehydrogenase (GDH). Glucose can be converted into pyruvate through glycolysis or used through the pentose phosphate pathway (PPP) to generate ribose 5‐phosphate (R5P) and NADPH. Some GC‐derived B‐cell lymphomas depend on PP2A and G6PD, a key PPP enzyme, to switch glucose carbon usage from glycolysis to the PPP. This provides antioxidant protection and supports ribonucleotide biosynthesis in proliferating cells. Pyruvate can also be converted into lactate as part of the “aerobic glycolysis” that tumor cells use to “bypass” the TCA cycle, to generate some ATP and to create biomass. This is known as the Warburg effect and can be induced by MYC and HIF1‐alpha stabilization in DLBCL and FL. Finally, mTORC1 activation in the GC happens downstream of T cell‐positive selection signals via the PI3K/Akt/mTOR pathway or downstream of nutrient signaling via activation of RagA/C and the v‐ATPase. These components either carry gain‐of‐function (GOF) mutations, are hyper‐activated, or are expressed at high levels in GC‐derived B‐cell lymphomas, resulting in mTORC1 constitutive activation. mTORC1 is the master regulator of anabolism and while constitutive activation is detrimental to GC B cells, it appears to favor lymphoma growth. G6PD, glucose‐6‐phosphate dehydrogenase; ROS, reactive oxygen species; v‐ATPase, vacuolar ATPase proton pump
