Abstract
Background
In two phase 3 trials, elobixibat, a locally acting ileal bile acid transporter inhibitor, resolved constipation and was well tolerated in Japanese patients with chronic constipation. We analyzed the efficacy, safety, and impact on quality of life (QOL) of elobixibat in patients with symptomatically more severe constipation in the two phase 3 trials.
Methods
This post hoc analysis of elobixibat treatment outcomes included data from a 2‐week, randomized, placebo‐controlled, phase 3 trial (10 mg/d), and a 52‐week, open‐label trial (5‐15 mg/d) in subgroups with severe constipation defined as ≤2 spontaneous bowel movements (SBMs) and ≤3 Bristol Stool Form Scale score during the second week of the 2‐week run‐in period. We also analyzed the rates of abdominal pain, diarrhea, and QOL in subgroups according to sex, presence of constipation‐predominant irritable bowel syndrome (IBS‐C) and side effects.
Key Results
In patients with severe constipation, there was significant improvement in the 10 mg elobixibat group compared to the placebo group in change in SBMs from baseline at week 1 (primary endpoint) of the 2‐week trial. The differences between groups were reduced in patients with more severe constipation. Increasing the dose to 15 mg was effective for more severe constipation in improving the number of SBMs per week in the 52‐week trial. Overall, elobixibat was well tolerated and improved QOL scores, irrespective of gender, presence of IBS‐C or side effects.
Conclusions & Inferences
Elobixibat is effective for symptomatically severe constipation, is well tolerated and improves QOL, irrespective of potentially confounding patient characteristics.
Keywords: abdominal pain, bile acid, constipation‐predominant irritable bowel syndrome, quality of life, spontaneous bowel movement
Key Points.
Elobixibat was effective and well tolerated for patients with chronic constipation in two Japanese phase 3 trials. We conducted post hoc analyses in patients with severe constipation in the trials.
Significant improvement in spontaneous bowel movements in the 10 mg elobixibat group was observed during week 1 (primary endpoint) compared with the placebo group.
Elobixibat was well tolerated and improved quality of life, irrespective of patient background or experience of side effects of abdominal pain and diarrhea.
1. INTRODUCTION
Constipation is self‐reported by 27.2% of North American adults1 and 28.4% of Japanese adults,2 although the prevalence differs with the definition used. The symptoms of chronic constipation are infrequent bowel movements, straining, a sensation of incomplete evacuation, and the presence of hard stools.3 These negatively affect quality of life (QOL) and impose socioeconomic burdens.4 In the absence of rectal evacuation disorders, most patients with constipation have normal colonic transit, but a few have slow colonic transit5 associated with reduced colonic propagated contractions.6 Lower levels of 48‐hour fecal excretion of total and secretory bile acids (BAs), such as deoxycholic and chenodeoxycholic acids, are associated with constipation.7 Total fecal BA excretion and fasting serum 7‐α‐hydroxy‐cholesten‐one (a surrogate marker of BA synthesis) have also been associated with slow colonic transit.8
Elobixibat (PubChem CID: 9939892) is a minimally absorbed inhibitor of the ileal BA transporter (IBAT, also called apical sodium‐dependent BA transporter). Approved in Japan in January 2018 for the treatment of chronic constipation, this IBAT inhibitor interrupts the enterohepatic circulation of BAs and upregulates hepatic BA synthesis.9 Increased concentrations of BAs in the colon then enhance colonic transit by stimulating fluid and electrolyte secretion10 and by inducing high‐amplitude propagated contractions (HAPCs), as demonstrated experimentally by the effects of intraluminal chenodeoxycholate.11 In phase 2 clinical trials in North America, elobixibat significantly accelerated colonic transit and improved bowel function12, 13; in dogs, it successfully induced HAPCs.14
Complementing the existing research, our research groups have recently published the results of phase 1, 2, and 3 trials in Japan.15, 16, 17 Among these, there have been two phase 3 trials sharing similar methodologies. The first was a randomized, double‐blind, placebo‐controlled, 2‐week trial in Japanese patients with chronic constipation, which showed that 10 mg of elobixibat once daily was safe and effective. The second was an open‐label, single‐arm, 52‐week trial, which showed that 5‐15 mg of elobixibat once daily was well tolerated, safe and improved QOL from baseline, and the most common adverse drug reactions (ADRs) of elobixibat were mild diarrhea or mild abdominal pain.
Previously, it was reported that experiencing <2 bowel movements per week or having a mean stool consistency of <3 on the Bristol Stool Form Scale (BSFS) score for 5 days could be used as independent predictors of slow transit constipation in Western cohorts.18, 19 In addition, recent reports in Eastern cohorts demonstrated that ≤2 bowel movements and ≤3 mean BSFS score for 5 days could be a valid predictive marker for slow colonic transit.20 To date, however, there has been no research to indicate whether elobixibat is effective in cases of more severe constipation that may be associated with slow transit constipation.
In this study, we aimed to assess the efficacy, safety and impact on QOL of elobixibat in specific patient subgroups, particularly focusing on baseline characteristics of severe constipation (defined as ≤2 spontaneous bowel movements [SBM] and ≤3 mean BSFS score measured in the second week of the 2‐week run‐in period), sex, and presence of features consistent with constipation‐predominant irritable bowel syndrome (IBS‐C).
2. MATERIALS AND METHODS
2.1. Study design
This post hoc analysis was based on previously reported data from the 2‐week, randomized, controlled trial (JapicCTI‐153062, or the “2‐week trial”) and the 52‐week, open‐label trial (JapicCTI‐153061, or the “52‐week trial”), the designs of which have been described in detail previously.17 In both trials, there was a 2‐week run‐in period before the eligible patients took the study drug for the study duration as an oral tablet before breakfast, once per day. Patients were monitored in an outpatient setting at each study site. In the 2‐week trial, 10 mg elobixibat or a placebo was taken once per day for 2 weeks (single cycle). In the 52‐week trial, participants received elobixibat oral tablets for 52 weeks at a dose of 10 mg/d for the first week; thereafter, patients could titrate the dose to 5 or 15 mg/d, or maintain the 10 mg/d dose, based on the effectiveness of the drug and the development of ADRs.
2.2. Participants
Males and females (non‐pregnant), aged ≥20 years, were included in the study if they satisfied the Rome III criteria for the diagnosis of functional constipation.21 Patients with IBS‐C were included in both trials. Patients were excluded if their chronic constipation was caused by organic disorders of the intestine (mechanical obstruction or neurological, endocrine, or metabolic disorders), medications, or intestinal or rectal surgery (except for simple appendectomy). Finally, participants in the 2‐week trial were excluded from the 52‐week, open‐label trial.
2.3. Post hoc analysis of efficacy
All efficacy analyses, including post hoc analyses, were based on a modified intention‐to‐treat population (ie, defined as patients who received at least one dose of the study drug).17 Subgroup analysis of efficacy was performed among subgroups by IBS‐C diagnosis (with and without), sex, age (<65 and ≥65 years), or more severe constipation. This final criterion was classified into three groups based on the occurrence of SBMs and mean BSFS score during the second week of the 2‐week run‐in period: severe constipation with SBM ≤2 and BSFS score ≤3; very severe constipation with SBM ≤1 and BSFS score ≤3; or absolute constipation if SBM = 0. We used modified SBM and BSFS score criteria from the previous report20 and assessed symptoms over a more rigorous period of 7 days rather than 5 days.
The efficacy endpoints were the same as previously reported.17 The primary endpoint of the 2‐week trial was the change from baseline (the second week of the 2‐week run‐in period) in the frequency of SBMs during the first week of treatment (week 1). Analyses were grouped by SBM number plus BSFS score, age, sex, and IBS‐C diagnosis. The prespecified secondary endpoints were change in frequency of complete SBMs (CSBMs; ie, SBMs associated with a sense of complete evacuation), proportions of weekly SBM and CSBM responders (defined as three or more SBMs or CSBMs per week and an increase of at least one SBM or CSBM per week from baseline), time to the first SBM, and so on. As a post hoc secondary endpoint, we included time to first CSBM.17 The subgroup post hoc analyses of efficacy were not prespecified except for prespecified subgroup analysis of the primary endpoint among subgroups by IBS‐C diagnosis, sex, and age.
In the 52‐week trial, data were recorded for each treatment week and at baseline. No treatment was received during the 2‐week run‐in period in either study.
2.4. Post hoc analysis of safety and QOL
The safety analyses relied on the data for all patients who received at least one dose of the study drug. In the 52‐week trial, the primary outcome was safety based on the presence of ADRs, with mild abdominal pain and diarrhea being most common. For the post hoc analysis of safety, we analyzed subgroups based on age, sex, and IBS‐C diagnosis to identify differences in the incidence (%), the median number of days to first onset, and the median number of days to resolution of abdominal pain and diarrhea.
The efficacy endpoint in the 52‐week trial also included an assessment of health‐related QOL, based on the Japanese version of the Patient Assessment of Constipation Quality of Life Questionnaire (JPAC‐QOL). The JPAC‐QOL contains 28 items grouped into four subscales covering the following: worries and concerns (11 items), physical discomfort (four items), psychosocial discomfort (eight items), and satisfaction (five items). For the post hoc analyses of the JPAC‐QOL, we analyzed subgroups based on age, sex, IBS‐C diagnosis and side effects (patients who experienced abdominal pain or diarrhea at least once). Assessments were performed at baseline, at weeks 4, 12, 24, 36, and 52, and at the time of patient withdrawal.
2.5. Statistical analysis
For the primary endpoint (changes in SBM frequency) and changes in CSBM frequency in the first week of the 2‐week trial, differences in the least squares means with 95% confidence intervals (CIs) between elobixibat and placebo were estimated using analysis of covariance (ANCOVA) model; models with different variances were used in the elobixibat and placebo groups. Odds ratios (OR) with 95% CIs for the proportions of SBM or CSBM responder rates in the elobixibat group vs placebo group were calculated using Firth's penalized likelihood logistic regression model. Hazard ratios (HR) with 95% CIs for the time to first SBM or CSBM in the elobixibat group vs the placebo group were estimated using Cox regression model with the same effects as logistic regression. Interaction terms of treatment by subgroup were included in each model, and tests of interaction among subgroups were also performed. The median times to first SBM were assessed by the Kaplan‐Meier method.
In the 52‐week trial, the numbers and proportions of patients who had abdominal pain or diarrhea were summarized, and we assessed the differences with 95% CIs between subgroups. The 95% CIs for risk difference in ADRs were calculated using Newcombe's method. Statistical comparison of the QOL subscale scores from baseline was performed using t test, and the mean differences with 95% CIs were calculated for the overall JPAC‐QOL scores of each subgroup during the 52‐week treatment period.
All P‐values were based on two‐sided tests, and the significance level was set at 0.05. All data were analyzed using SAS 9.4 (SAS Institute Inc, Cary, NC, USA) and were provided by EA Pharma Co., Ltd.
3. RESULTS
3.1. Baseline bowel movements
Table 1 shows the SBMs or CSBMs baseline characteristics of the included populations or subgroups that satisfied the criteria for severe constipation in the second week of the 2‐week run‐in period before the 2‐week and 52‐week trials. The data of the included participants were derived from those previously reported.17 As shown, both SBMs and CSBMs between placebo and elobixibat in each subgroup were well balanced except for CSBMs in patients with not severe constipation.
Table 1.
Baseline of the two phase 3 trials included for post hoc analysis
| 2‐wk triala | 52‐wk triala | |||||
|---|---|---|---|---|---|---|
| Placebo | Elobixibat 10 mg | Elobixibat 5‐15 mg | ||||
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | |
| SBMs/weekb | ||||||
| All | 63 | 1.7 (1.0) | 69 | 1.8 (0.9) | 340 | 1.5 (1.0) |
| Severe constipation | 47 | 1.4 (0.8) | 42 | 1.3 (0.7) | 264 | 1.2 (0.8) |
| Very severe constipation | 20 | 0.6 (0.5) | 22 | 0.7 (0.5) | 153 | 0.6 (0.5) |
| Absolute constipation | 8 | 0.0 (0.0) | 7 | 0.0 (0.0) | 63 | 0.0 (0.0) |
| Not severe constipation | 16 | 2.6 (1.0) | 27 | 2.5 (0.7) | 76 | 2.5 (0.9) |
| Not very severe constipation | 43 | 2.2 (0.6) | 47 | 2.3 (0.6) | 187 | 2.2 (0.6) |
| Not absolute constipation | 55 | 1.9 (0.8) | 62 | 2.0 (0.7) | 277 | 1.8 (0.7) |
| CSBMs/weekb | ||||||
| All | 63 | 0.5 (0.8) | 69 | 0.6 (0.8) | 340 | 0.4 (0.7) |
| Severe constipation | 47 | 0.2 (0.5) | 42 | 0.4 (0.7) | 264 | 0.3 (0.6) |
| Very severe constipation | 20 | 0.2 (0.4) | 22 | 0.2 (0.4) | 153 | 0.1 (0.3) |
| Absolute constipation | 8 | 0.0 (0.0) | 7 | 0.0 (0.0) | 63 | 0.0 (0.0) |
| Not severe constipation | 16 | 1.3 (1.2) | 27 | 0.8 (1.0) | 76 | 0.9 (0.9) |
| Not very severe constipation | 43 | 0.6 (1.0) | 47 | 0.7 (0.9) | 187 | 0.7 (0.8) |
| Not absolute constipation | 55 | 0.5 (0.9) | 62 | 0.6 (0.9) | 277 | 0.5 (0.8) |
Data show mean (SD).
Severe constipation: ≤2 SBM and ≤3 BSFS score per week, very severe constipation: ≤1 SBM and ≤3 BSFS score per week, absolute constipation: SBM = 0 per week in second run‐in week.
BSFS, Bristol Stool Form Scale; CSBM, complete spontaneous bowel movement; SBM, spontaneous bowel movement; SD, standard deviation.
The 2‐wk trial (10 mg dose only) was randomized and controlled, while the 52‐wk trial (10 mg starting dose, titrated between 5 mg and 15 mg) was open‐label.
Baseline value is based on the second run‐in week (week −1).
3.2. Post hoc analysis of efficacy in patients with severe constipation
In the 2‐week trial, significant improvements in the primary endpoint (change in SBMs from baseline at week 1) were observed in differences between the 10 mg elobixibat group and the placebo group in the subgroups except for absolute constipation (Figure 1A). The efficacy results for the severe constipation subgroup were similar to the efficacy in the total patient cohort. The differences between elobixibat and placebo groups were reduced in patients with more severe constipation. Thus, differences between elobixibat and placebo (95% CI; lower limit, upper limit) for severe constipation/very severe constipation/absolute constipation were 4.9 (3.3, 6.5), 3.8 (1.6, 6.0) and 1.7 (−2.3, 5.7), respectively. Regardless of the presence of IBS‐C symptoms, gender, or age, patients exhibited significant improvements in the primary endpoint.
Figure 1.
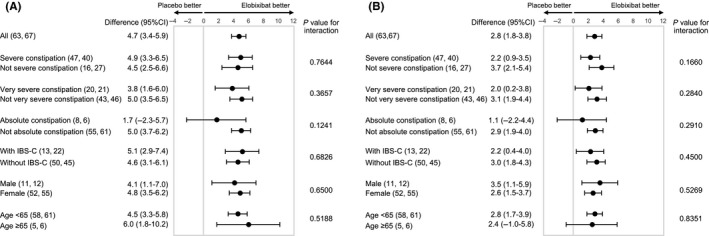
Subgroup analysis of elobixibat and placebo of changes in SBMs (A) and CSBMs (B) from baseline at week 1 in the 2‐wk randomized trial. Data show least‐squares mean differences (95% CI: lower limit‐upper limit) between placebo and elobixibat groups. The primary endpoint was the change in the frequency of SBMs at week 1. Number of patients in each subgroup are shown in parentheses (placebo group: elobixibat group). P values for interaction between closest subgroups of patients are shown in the table. ≤2 SBM and ≤3 BSFS score per week, very severe constipation: ≤1 SBM and ≤3 BSFS score per week, absolute constipation: SBM = 0 per week in second run‐in week. BSFS, Bristol Stool Form Scale; CI, confidence interval; CSBM, complete spontaneous bowel movement; IBS‐C, constipation‐predominant irritable bowel syndrome; SBM, spontaneous bowel movement
Similar results were observed for the change in CSBM from baseline to week 1 (Figure 1B). There were significant ORs of weekly SBM/CSBM responder rates at week 1 in different constipation severity subgroups except for absolute constipation (Figure 2A,B). For HRs of time to first CBM/CSBM, similar values were observed regardless of the constipation severity except for absolute constipation (Figure 2C,D). All tests of interaction were not statistically significant in all subgroups.
Figure 2.
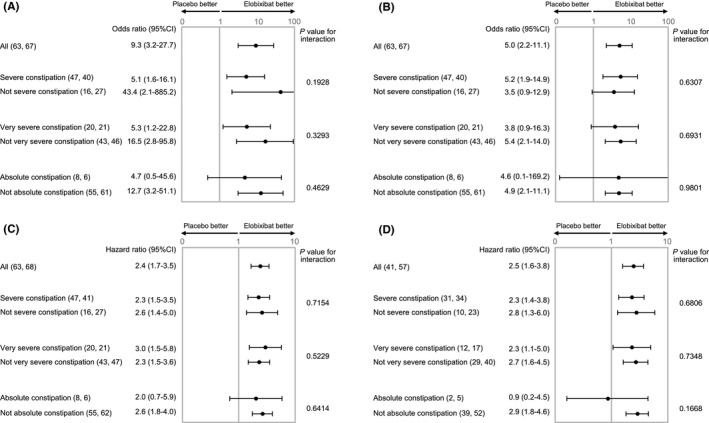
Subgroup analysis of elobixibat and placebo of weekly SBM (A) or CSBM (B) responder rates at week 1 or time to first SBM (C) or CSBM (D) in more severe chronic constipation in the 2‐wk randomized trial. Data show odds ratios (95% CI: lower limit‐upper limit) for the proportions SBM (A) or CSBM (B) responder rate in elobixibat group vs placebo group or hazard ratio (95% CI) for time to first SBM(C)/CSBM (D) between groups. Number of patients in each subgroup are shown in parentheses (placebo group: elobixibat group). P values for interaction between closest subgroups of patients are shown in the table. Severe constipation: ≤2 SBM and ≤3 BSFS score per week, very severe constipation: ≤1 SBM and ≤3 BSFS score per week, absolute constipation: SBM = 0 per week in second run‐in week. BSFS, Bristol Stool Form Scale; CI, confidence interval; CSBM, complete spontaneous bowel movement; SBM, spontaneous bowel movement
The median time to first SBM after elobixibat was similar between the total cohort and the severe constipation subgroup (5.1 and 5.6 hours, respectively), and was also similar for the total cohort and severe constipation subgroup treated with placebo (25.5 and 25.0 hours, respectively). In the very severe constipation subgroup, the median time to first SBM after elobixibat was 5.0 hours, which was significantly faster than the placebo treatment group (46.0 hours) and comparable to the entire constipation cohort and the severe constipation group.
Numbers needed to treat (NNTs) were calculated for the SBM and CSBM responder rates at week 1. NNTs for the SBM/CSBM response in the total constipation cohort were 2.9/2.9, severe constipation subgroup 3.6/3.1, and very severe constipation subgroup 2.8/3.6.
In the 52‐week trial, the mean weekly change in SBMs and CSBMs from baseline increased consistently in the severe, very severe, and absolute constipation subgroups, which were comparable to the changes observed in the whole constipation cohort (Figure 3). In patients with IBS‐C or without IBS‐C, the mean weekly change in SBMs and CSBMs from baseline increased equally, irrespective of IBS‐C status. A summary of treatment titration between the 5, 10 and 15 mg doses over the 52 weeks is shown in Table 2. As the severity of the baseline constipation increased, more patients titrated to the elobixibat 15 mg dose, the treatment duration at the 15 mg dose increased, and the proportion of patients still taking that dose in the last 4 weeks of the 52‐week trial increased.
Figure 3.
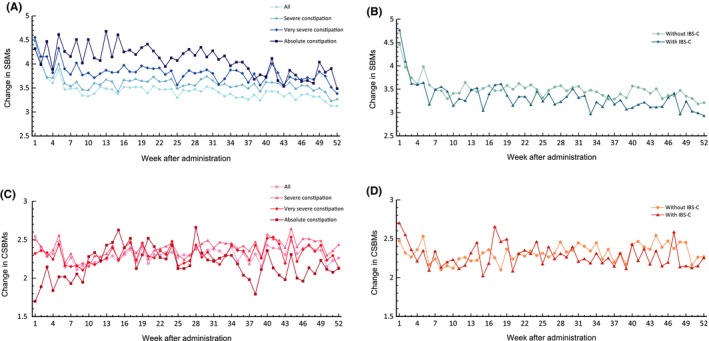
Mean changes in SBMs (A, B) and CSBMs (C, D) from last week of run‐in period (week −1) during the 52‐wk open‐label trial. Definitions: Severe constipation: ≤2 SBM and ≤3 BSFS score per week, very severe constipation: ≤1 SBM and ≤3 BSFS score per week, absolute constipation: SBM = 0 per week in second run‐in week. BSFS, Bristol Stool Form Scale; CSBM, complete spontaneous bowel movement; IBS‐C, constipation‐predominant irritable bowel syndrome; SBM, spontaneous bowel movement
Table 2.
Summary of treatment titration in the 52‐week open‐label trial
| Patient proportion in the 52‐wka, % (n) | Patient proportion in the last 4 wka, % (n) | Treatment duration in the 52‐wk, mean days (SD) | |
|---|---|---|---|
| All | |||
| 5 mg | 43 (145) | 31 (89) | 202 (138) |
| 10 mg | 100 (340) | 34 (99) | 133 (142) |
| 15 mg | 46 (157) | 37 (107) | 210 (136) |
| Severe constipation | |||
| 5 mg | 40 (105) | 28 (63) | 201 (138) |
| 10 mg | 100 (264) | 33 (75) | 128 (141) |
| 15 mg | 50 (132) | 41 (93) | 220 (137) |
| Very severe constipation | |||
| 5 mg | 36 (55) | 20 (26) | 178(139) |
| 10 mg | 100 (153) | 29 (37) | 113(135) |
| 15 mg | 58 (88) | 51 (66) | 237(131) |
| Absolute constipation | |||
| 5 mg | 13 (8) | 6 (3) | 116 (130) |
| 10 mg | 100 (63) | 21 (11) | 94 (128) |
| 15 mg | 83 (52) | 73 (38) | 242 (137) |
Severe constipation: ≤2 SBM and ≤3 BSFS score per week, very severe constipation: ≤1 SBM and ≤3 BSFS score per week, absolute constipation: SBM = 0 per week in second run‐in week.
BSFS, Bristol Stool Form Scale; SBM, spontaneous bowel movement; SD, standard deviation.
Double counts were allowed if the 340 patients, who fulfilled with modified intent to treat, changed the dose during 52 wk or the last 4 wk of 52 wk. In the 52‐wk trial, participants received elobixibat oral tablets for 52 wk at a dose of 10 mg/d for the first week; thereafter, patients could titrate the dose to 5 or 15 mg/d, or maintain the 10 mg/d dose, based on the effectiveness of the drug and the development of adverse drug reactions.
3.3. Post hoc analysis of safety and QOL in patients with severe constipation
Table 3 shows the safety summary, focusing on abdominal pain and diarrhea, as reported in the 52‐week trial. There were no significant differences in the frequency of either abdominal pain or diarrhea in relation to IBS‐C or sex. Among patients aged ≥65 years, only one experienced abdominal pain and only three experienced diarrhea. Excluding males and patients aged ≥65 years, due to small sample sizes, the median times to the first onset of abdominal pain and diarrhea were 1‐3 and 17‐28 days, respectively; the corresponding median times to resolution were 15‐17 and 5‐8 days, respectively.
Table 3.
Subgroup analysis of the incidence of abdominal pain and diarrhea in the 52‐wk open‐label trial
| Category | Subgroup (n) | Abdominal pain | ||
|---|---|---|---|---|
| Incidence, % (n) | Median first onset, day (IQR) | Median resolution, day (IQR) | ||
| Whole | All (340) | 24 (82) | 2 (1‐54) | 15 (7‐54) |
| Age | <65 (314) | 26 (81) | 2 (1‐54) | 15 (7‐54) |
| ≥65 (26) | 4 (1) | 1 | 4 | |
| Difference, % (95% CI) | 22 (6, 28) | ‐ | ‐ | |
| Sex | Male (57) | 19 (11) | 32 (1‐132) | 15 (3‐51) |
| Female (283) | 25 (71) | 2 (1‐49) | 17 (7‐57) | |
| Difference, % (95% CI) | −6 (−16, 7) | ‐ | ‐ | |
| IBS‐C | With (101) | 29 (29) | 3 (1‐49) | 15 (7‐33) |
| Without (239) | 22 (53) | 1 (1‐54) | 15 (7‐90) | |
| Difference, % (95% CI) | 7 (−3, 17) | ‐ | ‐ | |
| Category | Subgroup (n) | Diarrhea | ||
|---|---|---|---|---|
| Incidence, % (n) | Medium first onset, day (IQR) | Median resolution, day (IQR) | ||
| Whole | All (340) | 15 (50) | 21 (2‐86) | 6 (2‐34) |
| Age | <65 (314) | 15 (47) | 18 (2‐83) | 6 (2‐34) |
| ≥65 (26) | 12 (3) | 178 (2‐310) | 6 (1‐163) | |
| Difference, % (95% CI) | 3 (−14, 12) | ‐ | ‐ | |
| Sex | Male (57) | 16 (9) | 56 (8‐88) | 13 (4‐41) |
| Female (283) | 14 (41) | 18 (2‐79) | 6 (2‐33) | |
| Difference, % (95% CI) | 1 (−7, 13) | ‐ | ‐ | |
| IBS‐C | With (101) | 14 (14) | 28 (2‐88) | 5 (2‐47) |
| Without (239) | 15 (36) | 17 (4‐85) | 8 (3‐26) | |
| Difference, % (95% CI) | −1 (−9, 8) | ‐ | ‐ | |
Data show the number of patients % (n) for incidence, the difference % of the number of patients (95% CI: lower limit, upper limit) between groups, or the median day interquartile range (IQR) for the first onset or resolution.
CI, confidence interval; IBS‐C, constipation‐predominant irritable bowel syndrome; IQR, interquartile range.
Based on the sub‐score analysis of the JPAC‐QOL (Table 4A), elobixibat significantly improved physical discomfort, psychosocial discomfort, worries and concerns, satisfaction, and overall JPAC‐QOL scores compared with baseline. In the subgroup analysis of the overall JPAC‐QOL scores (Table 4B), no clinically significant differences were observed in relation to sex, IBS‐C status, or experience of abdominal pain or diarrhea as ADRs.
Table 4.
Results of (A) sub‐score comparison and (B) subgroup analysis of the JPAC‐QOL in the 52‐wk open‐label trial
| A. Sub‐score comparison (prespecified analysis) | |||||
|---|---|---|---|---|---|
| Evaluation (No. of patients) | JPAC‐QOL | ||||
| Overall | Physical discomfort | Psychosocial discomfort | Worries and concerns | Satisfaction | |
| Baseline (339) | 1.6 (0.6) | 1.9 (0.8) | 0.8 (0.7) | 1.4 (0.8) | 3.3 (0.5) |
| Week 4 (334) | 1.0 (0.6)* | 1.0 (0.8)* | 0.5 (0.5)* | 1.0 (0.6)* | 2.2 (1.0)* |
| Week 12 (320) | 0.9 (0.5)* | 0.9 (0.7)* | 0.5 (0.5)* | 0.9 (0.6)* | 2.0 (1.0)* |
| Week 24 (309) | 0.9 (0.6)* | 0.8 (0.7)* | 0.4 (0.5)* | 0.8 (0.6)* | 1.9 (1.2)* |
| Week 36 (300) | 0.8 (0.5)* | 0.8 (0.7)* | 0.4 (0.5)* | 0.8 (0.5)* | 1.8 (1.1)* |
| Week 52 (289) | 0.8 (0.6)* | 0.8 (0.7)* | 0.4 (0.4)* | 0.8 (0.6)* | 1.8 (1.1)* |
| B. Subgroup analysis (post hoc analysis) | |||||||
|---|---|---|---|---|---|---|---|
| Category | Subgroup (No. of patients) | Baseline | Overall JPAC‐QOL Score | ||||
| Week 4 | Week 12 | Week 24 | Week 36 | Week 52 | |||
| Whole | All (339) | 1.6 (0.6) | 1.0 (0.6) | 0.9 (0.5) | 0.9 (0.6) | 0.8 (0.5) | 0.8 (0.6) |
| Age | <65 (313) | 1.7 (0.6) | 1.1 (0.6) | 1.0 (0.5) | 0.9 (0.6) | 0.9 (0.5) | 0.8 (0.5) |
| ≥65 (26) | 1.4 (0.6) | 0.8 (0.4) | 0.7 (0.4) | 0.8 (0.5) | 0.8 (0.6) | 0.8 (0.6) | |
| Difference (95% CI) | 0.3 (0.01, 0.5) | 0.3 (0.06, 0.5) | 0.2 (0.01, 0.4) | 0.09 (−0.1, 0.3) | 0.07 (−0.2, 0.3) | 0.03 (−0.2, 0.3) | |
| Sex | Male (56) | 1.4 (0.4) | 1.0 (0.6) | 0.9 (0.5) | 0.8 (0.5) | 0.8 (0.5) | 0.8 (0.6) |
| Female (283) | 1.7 (0.6) | 1.1 (0.6) | 1.0 (0.5) | 0.9 (0.6) | 0.9 (0.5) | 0.9 (0.5) | |
| Difference (95% CI) | −0.3 (−0.4, −0.09) | −0.08 (−0.2, 0.08) | −0.02 (−0.2, 0.1) | −0.09 (−0.3, 0.08) | −0.1 (−0.3, 0.05) | −0.04 (−0.2, 0.1) | |
| IBS‐C | With (101) | 1.7 (0.6) | 1.1 (0.5) | 1.0 (0.5) | 0.9 (0.5) | 0.9 (0.4) | 0.9 (0.5) |
| Without (238) | 1.6 (0.6) | 1.0 (0.6) | 0.9 (0.6) | 0.9 (0.6) | 0.8 (0.5) | 0.8 (0.6) | |
| Difference (95% CI) | 0.1 (0.001, 0.3) | 0.1 (−0.02, 0.2) | 0.03 (−0.09, 0.2) | 0.03 (−0.1, 0.2) | 0.03 (−0.1, 0.2) | 0.06 (−0.08, 0.2) | |
| Abdominal pain | Witha (81) | 1.5 (0.5) | 1.1 (0.5) | 1.0 (0.5) | 1.0 (0.6) | 0.9 (0.5) | 0.9 (0.5) |
| Without (258) | 1.7 (0.6) | 1.0 (0.6) | 0.9 (0.5) | 0.9 (0.5) | 0.8 (0.5) | 0.8 (0.6) | |
| Difference (95% CI) | −0.1 (−0.3, 0.01) | 0.06 (−0.08, 0.2) | 0.07 (−0.07, 0.2) | 0.2 (0.002, 0.3) | 0.08 (−0.06, 0.2) | 0.07 (−0.08, 0.2) | |
| Diarrhea | Witha (49) | 1.6 (0.6) | 1.2 (0.6) | 1.0 (0.5) | 0.9 (0.6) | 0.9 (0.5) | 1.0 (0.7) |
| Without (290) | 1.6 (0.6) | 1.0 (0.5) | 0.9 (0.5) | 0.9 (0.6) | 0.8 (0.5) | 0.8 (0.5) | |
| Difference (95% CI) | −0.07 (−0.3, 0.1) | 0.1 (−0.04, 0.3) | 0.05 (−0.1, 0.2) | −0.04 (−0.2, 0.1) | 0.01 (−0.2, 0.2) | 0.1 (−0.04, 0.3) | |
Data show mean (SD) or difference (95% CI: lower limit, upper limit) between groups.
CI, confidence interval; IBS‐C, constipation‐predominant irritable bowel syndrome; JPAC‐QOL, Japanese version of the Patient Assessment of Constipation Quality of Life Questionnaire; SD, standard deviation.
The patient experienced at least one event over the 52 wk.
P < 0.0001 compared with baseline (t test).
4. DISCUSSION
In this post hoc analysis, we assessed the efficacy of elobixibat in patients with severe chronic constipation based on a combination of SBM and BSFS criteria, which may be a predictive marker of slow transit constipation. Elobixibat at a 10 mg dose produced significant improvements in SBM‐ and CSBM‐based endpoints in the 2‐week trial, although its efficacy decreased with increased baseline severity of constipation, as determined from the baseline frequency of SBMs. Long‐term improvements tended to be observed in patients with fewer SBMs at baseline, but these remained comparable to those in the total study population because the elobixibat dose could be increased to 15 mg if the effect was not sufficient at the lower dose.
We analyzed the data of 157 patients to evaluate the incidence of ADRs, specifically abdominal pain and diarrhea, from 2 weeks before to 2 weeks after the first dose increase to 15 mg; there was no increase in the incidence of ADRs after increasing the dose. In addition, the incidences of adverse events in the 5, 10, and 15 mg groups were not dose dependent in the Japanese phase IIb study,16 and from this post hoc analysis, elobixibat was well tolerated and improved the JPAC‐QOL score in each subgroup. In fact, most ADR reports were of mild severity, and the majority of patients recovered without titrating dose down and the JPAC‐QOL score still improved, implying that tolerability and safety were good.
Patients with and without IBS‐C showed similar improvements in the primary endpoint at week 1 in the 2‐week trial; these two groups also showed consistently higher frequencies of SBMs and CSBMs during the 52‐week trial. Moreover, elobixibat was well tolerated in terms of abdominal pain and diarrhea, which were observed with similar incidence in those with IBS‐C diagnosis compared to the rest of the cohort; similarly, there were no significant differences between the two groups in the JPAC‐QOL scores in the 52‐week trial. It has been speculated that the abdominal pain experienced by some while taking elobixibat might be different from that of IBS because elobixibat induces HAPCs,14 which might be associated with mass movements and short‐lived pain due to the temporary increase in colonic pressure. Other potential mechanisms for the abdominal pain may result from the increased colonic BAs, since BAs reduced rectal sensory thresholds in humans11 and increased mucosal permeability in rabbits.22 The mechanisms whereby elobixibat results in symptom relief in IBS should be a topic for future research.
An NNT analysis was provided to allow comparison with other drugs, but there are important considerations when interpreting this for chronic constipation.23, 24 For example, it is ideal that relief from chronic constipation by medication be observed as soon after administration as possible. In our study, we appraised NNT for weekly SBM and CSBM responder rates at week 1 for the entire patient cohort were both 3, and the NNTs ranged from 3 to 4 in the severe to very severe constipation groups. These data suggest that elobixibat is effective for both normal and slow transit constipation because of the rapid onset of efficacy.
Given that the mean 5‐day BSFS score of ≤3 provided 68.0% sensitivity, 69.7% specificity, and 69.4% accuracy, and that the stool frequency of ≤2 bowel movements in 5 days provided 64.0% sensitivity, 83.1% specificity, and 84.0% accuracy for predicting delayed colonic transit time,20 it is possible that the patients included in this subgroup analysis with severe constipation were mostly suffering slow transit constipation. However, this cannot be definitely concluded, since there was no formal measurement of colonic transit at baseline. In chronic constipation, the colonic transit time is delayed25 and the number and duration of mass movements are significantly reduced26 compared with healthy volunteers. Elobixibat is expected to correct low colonic BA levels by partly blocking the IBAT in the distal part of the small intestine and by normalizing BAs and inducing dual action of colonic motility and secretion. These effects can rectify the lower amplitudes and frequencies of colonic contractions, including HAPCs, in patients with slow transit constipation. Elobixibat may, therefore, benefit all patients with chronic constipation who have reduced HAPCs and delayed transit times.27
One of the limitations of this post hoc analysis was the small number of patients aged ≥65 years included in the two trials. Further studies are, therefore, necessary to evaluate the safety and efficacy of elobixibat for the treatment of chronic constipation in an elderly patient group. In addition, part of the data is based on an open‐label study with all its limitations.
In conclusion, elobixibat was effective for patients with severe constipation at a starting dose of 10 mg. Increasing the dose to 15 mg can benefit patients who have very few (<2) SBMs per week at baseline or on treatment with the 10 mg dose. Indeed, over 52 weeks of therapy, elobixibat was efficacious at increasing over baseline the number of SBMs and CSBMs, was well tolerated, and improved QOL, irrespective of the patient background (sex and concomitant IBS‐C) or experience of side effects of abdominal pain and diarrhea.
CONFLICT OF INTEREST
AN has served as a medical adviser to EA Pharma Co., Ltd. ST and SK are employees of EA Pharma Co., Ltd. PGG and JPM are employees of Albireo AB. MC has served as a consultant to EA Pharma Co., Ltd. and Albireo AB with no personal financial remuneration and consulting fees paid to his employer, Mayo Clinic. All authors have no competing interests.
AUTHOR CONTRIBUTIONS
AN and ST were involved in designing and interpretation of the post hoc analysis, and wrote a first draft and edited subsequent draft. SK was involved in performing of statistical post hoc analysis and edited subsequent draft. PGG and JPM were involved in interpretation of the post hoc analysis and edited subsequent draft. MC demonstrated concept of BA deficiency in constipation, providing rationale for IBAT inhibitor, clinical trial design; is senior corresponding author and did extensive editing of the manuscript. All authors had complete access to the data and reviewed and approved the final draft of the manuscript for submission.
ACKNOWLEDGMENT
The authors thank Mrs Cindy Stanislav for excellent secretarial assistance.
Nakajima A, Taniguchi S, Kurosu S, Gillberg P‐G, Mattsson JP, Camilleri M. Efficacy, long‐term safety, and impact on quality of life of elobixibat in more severe constipation: Post hoc analyses of two phase 3 trials in Japan. Neurogastroenterol Motil. 2019;31:e13571 10.1111/nmo.13571
Clinical Trials Registration Numbers: Japan Pharmaceutical Information Center numbers, JapicCTI‐153061 (http://rctportal.niph.go.jp/en/detail?trial_id=JapicCTI-153061) and JapicCTI‐153062 (http://rctportal.niph.go.jp/en/detail?trial_id=JapicCTI-153062).
Funding information
The two phase 3 trials were funded by EA Pharma Co., Ltd. and Mochida Pharmaceutical Co., Ltd. There is not any other study‐related funding including the conduct of the post hoc analysis. Dr M. Camilleri receives funding from the National Institutes of Health (R01‐DK67071 and R01‐DK115950).
REFERENCES
- 1. Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004;99:750‐759. [DOI] [PubMed] [Google Scholar]
- 2. Tamura A, Tomita T, Oshima T, et al. Prevalence and self‐recognition of chronic constipation: results of an internet survey. J Neurogastroenterol Motil. 2016;22:677‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150:1393‐1407. [DOI] [PubMed] [Google Scholar]
- 4. Sun SX, Dibonaventura M, Purayidathil FW, Wagner JS, Dabbous O, Mody R. Impact of chronic constipation on health‐related quality of life, work productivity, and healthcare resource use: an analysis of the National Health and Wellness Survey. Dig Dis Sci. 2011;56:2688‐2695. [DOI] [PubMed] [Google Scholar]
- 5. Nullens S, Nelsen T, Camilleri M, et al. Regional colon transit in patients with dys‐synergic defaecation or slow transit in patients with constipation. Gut. 2012;61:1132‐1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dinning PG, Zarate N, Hunt LM, et al. Pancolonic spatiotemporal mapping reveals regional deficiencies in, and disorganization of colonic propagating pressure waves in severe constipation. Neurogastroenterol Motil. 2010;22:e340‐e349. [DOI] [PubMed] [Google Scholar]
- 7. Shin A, Camilleri M, Vijayvargiya P, et al. Bowel functions, fecal unconjugated primary and secondary bile acids, and colonic transit in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2013;11:1270‐1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vijayvargiya P, Busciglio I, Burton D, Donato L, Lueke A, Camilleri M. Bile acid deficiency in a subgroup of patients with irritable bowel syndrome with constipation based on biomarkers in serum and fecal samples. Clin Gastroenterol Hepatol. 2018;16:522‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Acosta A, Camilleri M. Elobixibat and its potential role in chronic idiopathic constipation. Therap Adv Gastroenterol. 2014;7:167‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wingate DL, Krag E, Mekhjian HS, Phillips SF. Relationships between ion and water movement in the human jejunum, ileum and colon during perfusion with bile acids. Clin Sci Mol Med. 1973;45:593‐606. [DOI] [PubMed] [Google Scholar]
- 11. Bampton PA, Dinning PG, Kennedy ML, Lubowski DZ, Cook IJ. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am J Physiol Gastrointest Liver Physiol. 2002;282:G443‐G449. [DOI] [PubMed] [Google Scholar]
- 12. Wong BS, Camilleri M, McKinzie S, Burton D, Graffner H, Zinsmeister AR. Effects of A3309, an ileal bile acid transporter inhibitor, on colonic transit and symptoms in females with functional constipation. Am J Gastroenterol. 2011;106:2154‐2164. [DOI] [PubMed] [Google Scholar]
- 13. Chey WD, Camilleri M, Chang L, Rikner L, Graffner H. A randomized placebo‐controlled phase IIb trial of a3309, a bile acid transporter inhibitor, for chronic idiopathic constipation. Am J Gastroenterol. 2011;106:1803‐1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taniguchi S, Yano T, Imaizumi M, Manabe N. Elobixibat, an ileal bile acid transporter inhibitor, induces giant migrating contractions during natural defecation in conscious dogs. Neurogastroenterol Motil. 2018;30:e13448. [DOI] [PubMed] [Google Scholar]
- 15. Kumagai Y, Amano H, Sasaki Y, et al. Effect of single and multiple doses of elobixibat, an ileal bile acid transporter inhibitor, on chronic constipation: A randomized controlled trial. Br J Clin Pharmacol. 2018;84:2393‐2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nakajima A, Seki M, Taniguchi S. Determining an optimal clinical dose of elobixibat, a novel inhibitor of the ileal bile acid transporter, in Japanese patients with chronic constipation: a phase II, multicenter, double‐blind, placebo‐controlled randomized clinical trial. J Gastroenterol. 2018;53:525‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakajima A, Seki M, Taniguchi S, et al. Safety and efficacy of elobixibat for chronic constipation: results from a randomised, double‐blind, placebo‐controlled, phase 3 trial and an open‐label, single‐arm, phase 3 trial. Lancet Gastroenterol Hepatol. 2018;3:537‐547. [DOI] [PubMed] [Google Scholar]
- 18. Glia A, Lindberg G, Nilsson LH, Mihocsa L, Akerlund JE. Clinical value of symptom assessment in patients with constipation. Dis Colon Rectum. 1999;42:1401‐1408. [DOI] [PubMed] [Google Scholar]
- 19. Saad RJ, Rao SS, Koch KL, et al. Do stool form and frequency correlate with whole‐gut and colonic transit? Results from a multicenter study in constipated individuals and healthy controls. Am J Gastroenterol. 2010;105:403‐411. [DOI] [PubMed] [Google Scholar]
- 20. Jaruvongvanich V, Patcharatrakul T, Gonlachanvit S. Prediction of delayed colonic transit using Bristol stool form and stool frequency in Eastern constipated patients: a difference from the West. J Neurogastroenterol Motil. 2017;23:561‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480‐1491. [DOI] [PubMed] [Google Scholar]
- 22. Chadwick VS, Gaginella TS, Carlson GL, Debongnie JC, Phillips SF, Hofmann AF. Effect of molecular structure on bile acid‐induced alterations in absorptive function, permeability, and morphology in the perfused rabbit colon. J Lab Clin Med. 1979;94:661‐674. [PubMed] [Google Scholar]
- 23. Ford AC, Suares NC. Effect of laxatives and pharmacological therapies in chronic idiopathic constipation: systematic review and meta‐analysis. Gut. 2011;60:209‐218. [DOI] [PubMed] [Google Scholar]
- 24. Bharucha AE, Pemberton JH, Locke GR 3rd. American Gastroenterological Association technical review on constipation. Gastroenterology. 2013;144:218‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manabe N, Wong BS, Camilleri M, Burton D, McKinzie S, Zinsmeister AR. Lower functional gastrointestinal disorders: evidence of abnormal colonic transit in a 287 patient cohort. Neurogastroenterol Motil. 2010;22:293‐e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bassotti G, Gaburri M, Imbimbo BP, et al. Colonic mass movements in idiopathic chronic constipation. Gut. 1988;29:1173‐1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rao SS, Sadeghi P, Beaty J, Kavlock R. Ambulatory 24‐hour colonic manometry in slow‐transit constipation. Am J Gastroenterol. 2004;99:2405‐2416. [DOI] [PubMed] [Google Scholar]


