Abstract
Objective
To evaluate potential adverse associations of individual antiretroviral medications (ARVs) used in combination antiretroviral therapy regimens on cardiac structure and function in youth with perinatally-acquired HIV infection (PHIV).
Design
PHIV youth (n=325) enrolled in a prospective multisite cohort study had a single echocardiogram at age 7–16 years to evaluate cardiac function and structure.
Methods
We applied several statistical approaches to evaluate associations between use of 18 individual ARVs with Z-scores for 11 measures of left ventricular (LV) function and structure. These included simultaneously evaluating all ARVs in adjusted linear regression models controlling for the False Discovery Rate (FDR), applying hierarchical models to estimate individual ARV effects as deviations from drug class means, and evaluating latent measures of cardiac function and structure underlying multiple echocardiographic parameters.
Results
Youth taking combination regimens with a protease inhibitor (69%) had significantly better cardiac function than those on other regimens. After FDR control and adjustment for other ARVs, no individual ARV was significantly associated with any measure of LV function, but zidovudine was associated with higher adjusted mean Z-scores for one measure of LV structure (end-systolic wall stress). Factor analysis identified three latent factors: heart function, heart size, and heart wall stress. Lopinavir was associated with better heart function scores, while zidovudine was associated with higher wall stress scores. Zidovudine and nevirapine were associated with higher heart size factor scores.
Conclusions
Despite cardioprotective effects of combination regimens in PHIV youth, individual ARV medications were associated with altered cardiac structure, which could progress to symptomatic cardiomyopathy in adulthood.
Keywords: cardiac, left ventricular, perinatal HIV, children, latent variable, factor analysis, hierarchical models
INTRODUCTION
Perinatally HIV-infected (PHIV) children born early in the HIV epidemic often experienced severe cardiac-related conditions such as cardiomyopathy, congestive heart failure, and even cardiac-related deaths, but these conditions now rarely occur among those receiving combination antiretroviral treatment (cART) regimens [1–7]. However, cardiac structure and function in these youth differ subtly from that of youth who are perinatally HIV-exposed but uninfected (PHEU) [7]. These differences may be partially attributable to chronic inflammation, but may also be related to individual antiretroviral medications (ARVs) included in combination regimens [8]. These subclinical differences could lead to symptomatic cardiomyopathy or atherosclerotic heart disease as youth become adults and are exposed to other traditional cardiovascular risk factors such as smoking, obesity, and diabetes [3,7,8–10].
Several studies have examined the effects of HIV infection and ARV treatment on cardiac status in children. The Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV Infection Study (P2C2 Study) conducted in the era before cART (1990–1997) found that increased left ventricular (LV) mass and decreased LV contractility were early predictors of mortality in PHIV children [2–4,11]. More recently, the Pediatric HIV/AIDS Cohort Study (PHACS) network measured cardiac structure and function in 325 PHIV and 189 PHEU youth, and compared these measures to those of 70 P2C2 PHIV youth in the same age range [7]. Measures of cardiac function, such as LV fractional shortening and contractility, were significantly and substantially poorer in the P2C2 youth than in the current PHIV and PHEU AMP cohorts. A longer duration of antiretroviral treatment (ART) was associated with lower fractional shortening in the P2C2 cohort but not in the more contemporary AMP PHIV cohort, who had typically received cART since infancy [7]. Recent studies clearly demonstrate that the use of cART is associated with better cardiac functioning among PHIV youth [12].
Despite the cardioprotective effect of cART on today’s PHIV youth, the possibility of adverse effects of individual ART drugs on cardiac structure and function has received little attention. Studies in adults with HIV have found that abacavir and protease inhibitors (PIs) are associated with clinical cardiac outcomes such as myocardial infarction and atherosclerotic heart disease [13–15], but these findings are based on very large studies with long-term follow-up and in age groups with higher incidence of cardiovascular events. Several animal and human studies have evaluated effects of zidovudine exposure during pregnancy on cardiotoxicity and cardiac congenital anomalies [16–22], but only a few have evaluated zidovudine’s effect on cardiac function among cART-treated children and adolescents [5].
Evaluating the association of individual ARVs with cardiac outcomes in PHIV youth is complicated by possible drug interactions, as well as by the large number of regimens currently used. The standard approach of evaluating individual ARVs one at a time can be biased because it does not account for other drugs in the same regimen [23]. In addition, analyzing a large number of echocardiographic measures results in multiple statistical comparisons which must be considered when interpreting results. In this study, we applied several novel statistical approaches to detect possible adverse effects of individual ARVs on echocardiographic measures of cardiac structure and function among PHIV youth enrolled in the PHACS Adolescent Master Protocol (AMP).
METHODS
Study Population
AMP is a prospective cohort study conducted at 14 academic research hospitals in the US and Puerto Rico to evaluate the impact of HIV infection and ART on the development of children with perinatal HIV infection [7]. The AMP Study includes both PHIV and PHEU children, aged 7 to 16 years at enrollment between March 2007 and November 2009. As part of the study, enrolled children had a single echocardiogram conducted between 2008 and 2010. The study protocol was approved by the institutional review board (IRB) of each participating site and the Harvard T.H. Chan School of Public Health, and written informed consent was obtained from each child’s parent or legal guardian and assent from older participants as specified by the local IRB.
Our analysis included only the AMP PHIV children, who had generally received many years of cART. Children were excluded if they had congenital cardiac abnormalities, such as a large atrial septal defect or a ventricular septal defect. At each semi-annual AMP study visit, information about participants and their families was gathered through clinical interviews and chart reviews. Health status was ascertained at study entry and at each study visit through physical and laboratory evaluations and chart abstraction, and included growth measures (height, weight, and body mass index [BMI], in kg/m2), CD4 counts and HIV-1 RNA viral load (VL) measures, and Centers for Disease Control and Prevention (CDC) clinical disease class. Information on ART regimens was obtained since ART initiation, with start and stop dates for each regimen; cART was defined as any regimen that included three or more ARVs from two or more drug classes.
Echocardiographic Assessment of Cardiac Structure and Function
Trained study staff obtained a single M-mode and 2-dimensional echocardiogram on each child using a standardized protocol with concurrent blood pressure and heart rate measurements. To improve reliability, all echocardiograms were centrally re-measured at the echocardiographic core laboratory at Boston Children’s Hospital. Echocardiographic Z scores were calculated using normative data from an established reference cohort of healthy children from Boston Children’s Hospital, with adjustment for age and body-surface area as appropriate [24]. Although a large number of echocardiographic parameters were measured, we focused on three primary measures of left ventricular (LV) function (ejection fraction, contractility, and fractional shortening), four primary measures of LV structure (LV mass, end-diastolic [ED] dimension, ED posterior wall thickness, and end-systolic [ES] wall stress), and four secondary measures of LV structure (LV ES and ED volumes, ES thickness-to-dimension ratio, and ED septal thickness). The study was designed to obtain echocardiograms on 500 PHIV and PHEU children to provide 80% power for detecting clinically meaningful differences in mean Z-scores, and echocardiograms were no longer conducted after December 2010, when this target sample size was achieved.
Statistical Methods
We summarized ART exposures as the percent of youth currently exposed and cumulative years of exposure to each ARV at echocardiography, and as the percent of youth receiving certain pairs of ARVs within their combination regimen.
We utilized several approaches to identify potential associations between ART medications and cardiac echocardiographic measures. We first applied the “standard” approach of separately evaluating each ARV one at a time with each cardiac outcome using linear regression models adjusting for age and BMI Z-score at the time of echocardiogram as well as sex, race, and ethnicity. One set of models was fit for current ARVs (exposed vs unexposed) and another for cumulative duration of ARVs. As noted above, estimated associations for each individual ARV may be biased when not accounting for other ARVs in the same regimen. Thus, we also fit linear regression models simultaneously including all individual ARVs to reduce such potential bias; these estimates are referred to as “mutually-adjusted” results. Given the large number of statistical comparisons made (for combinations of18 ARVs with 11 echocardiographic measures), significance testing was based on controlling the false discovery rate (FDR) using a Benjamini and Liu threshold of 0.10 within each echocardiographic parameter [25–26].
A model with so many correlated ARV exposures could lead to multicollinearity and unstable estimates with inflated variances; thus, we also used a hierarchical model including fixed effects for each drug class and specifying a random effect for each drug within its corresponding drug class, as described elsewhere [23]. This hierarchical model essentially estimates individual drug effects as the drug class mean plus a deviation for the individual drug, and generally performs better than separate models and mutually-adjusted models; in particular, there are fewer false positives and estimates are generally less biased and more precise [23].
We also evaluated associations between individual ARVs and cardiac outcomes using approaches that accounted for the strong correlations among the echocardiographic measures and reduced the dimension of outcomes to a smaller set of latent variables. We conducted an exploratory factor analysis to identify correlated clusters of echocardiographic Z-score measures, and then used the resulting standardized factor scores as latent outcomes, again evaluating all individual ARVs simultaneously in a single model for each latent factor. Factors were selected based on scree plots, eigenvalues, and percent of explained variance. Before conducting the factor analysis, missing outcome Z-scores for a small percentage of youth were imputed using an expectation-maximization (EM) algorithm [27]. As an alternative latent variable approach, we fit separate repeated measures models for each “set” of primary echocardiographic outcomes, with an unstructured covariance assumption to account for correlation between different cardiac outcomes measured on the same participant [28]. More specifically, we fit a repeated measures model for the functional echocardiographic parameters including fractional shortening, ejection fraction, and contractility that assumed a constant effect of each ARV drug on all three parameters, although an effect for outcome was included to allow the background mean z-scores to vary over the three parameters. All ARV drugs were included in a single model. Similarly, we fit a repeated measures model including LV mass, ED dimension, and ES volume, assuming a constant effect of each ARV drug on these “heart size” measures. Last of all, we fit a repeated measures model for parameters reflecting heart wall stress, including ES wall stress, thickness-to-dimension ratio, ED wall thickness, and ED septal thickness.
Finally, sensitivity analyses were conducted to evaluate how estimated associations changed when adjusted for HIV disease severity, including low nadir CD4% (<15%), unsuppressed VL (>5000 copies/mL) at the time of echocardiography, and prior AIDS diagnosis (CDC Class C). Analyses were conducted using SAS (Version 9.4), and were based on data submitted as of July 2014. Assumptions of models were examined and verified using standard model diagnostics and influence statistics for mixed effect models [28,29].
RESULTS
Participant Characteristics and ART Exposures
Of the 451 PHIV youth enrolled in the PHACS AMP Study, 325 (72%) had an echocardiogram, obtained at a mean (SD) age of 13.0 (2.7) years (range 7.0–18.7). Of these, 47% were male, 66% were non-Hispanic black, and 23% were Hispanic. The youth were relatively healthy at the time of echocardiography, with a median CD4 count of 693 cells/μl and most having suppressed VL (69% with VL<400 copies/mL); however, 25% had a prior AIDS-defining diagnosis (CDC Class C). Additional details on this cohort have been reported previously [7]. Youth with echocardiograms were demographically similar to those without echocardiograms (data not shown).
At the time of echocardiography, 88% were receiving cART (69% with a PI), and the mean cumulative duration on cART was 8.1 years (Table 1). A total of 137 different cART regimens were reported at the time of echocardiography. The most common nucleoside reverse transcriptase inhibitors (NRTIs) were lamivudine, zidovudine, tenofovir disoproxil fumarate (TDF), and abacavir. Among non-nucleoside reverse transcriptase inhibitors (NNRTIs), efavirenz was used more often than nevirapine, and ritonavir-boosted lopinavir was the most common PI (Table 1). Commonly used NRTI combinations at the time of echocardiography included zidovudine plus lamivudine (26%), TDF plus emtricitabine (20%), and abacavir plus lamivudine (18%).
Table 1.
Summary of Antiretroviral Drug Exposures for 325 Perinatally HIV-infected Youth in the PHACS Adolescent Master Protocol (AMP) Study with Echocardiograms
| Drug Class | Antiretroviral drug | N (%) ever on drug (%) | N (%) on drug at echocardiogram | Mean cumulative duration (years) | 75th percentile (years) | Maximum duration (years) |
|---|---|---|---|---|---|---|
| Regimen | cART | 314 (96.9) | 284 (87.7) | 8.12 | 10.58 | 13.22 |
| cART with PI | 290 (89.5) | 223 (68.8) | 6.92 | 10.07 | 13.11 | |
| NRTIs | Lamivudine (3TC) | 301 (92.6) | 162 (50.0) | 5.75 | 8.76 | 13.52 |
| Abacavir (ABC) | 127 (39.1) | 87 (26.9) | 1.46 | 1.95 | 11.15 | |
| Stavudine (d4T) | 244 (75.1) | 55 (17.0) | 4.58 | 7.49 | 13.86 | |
| Didanosine (ddI) | 216 (66.5) | 64 (19.8) | 3.31 | 5.96 | 14.71 | |
| Emtricitabine (FTC) | 91 (28.0) | 75 (23.1) | 0.58 | 0.28 | 7.88 | |
| Tenofovir (TDF) | 107 (32.9) | 91 (28.1) | 0.71 | 0.81 | 7.23 | |
| Zidovudine (ZDV) | 287 (88.3) | 107 (33.0) | 4.27 | 7.41 | 13.86 | |
| NNRTIs | Efavirenz (EFV) | 129 (39.7) | 66 (20.4) | 1.52 | 2.15 | 10.60 |
| Nevirapine (NVP) | 113 (34.8) | 18 (5.6) | 1.29 | 1.08 | 11.69 | |
| PIs | Amprenavir (APV) | 23 (7.1) | 1 (0.3) | 0.20 | 0.00 | 7.38 |
| Atazanavir (ATV) | 58 (17.8) | 42 (13.0) | 0.39 | 0.00 | 7.43 | |
| Darunavir (DRV) | 17 (5.2) | 13 (4.0) | 0.06 | 0.00 | 3.50 | |
| Indinavir (IDV) | 20 (6.2) | (None) | 0.14 | 0.00 | 7.35 | |
| Lopinavir (LPV/r) | 173 (53.2) | 123 (38.0) | 2.44 | 5.18 | 9.73 | |
| Nelfinavir (NFV) | 197 (60.6) | 36 (11.1) | 2.82 | 5.24 | 12.96 | |
| Ritonavir (RTV) | 235 (72.3) | 58 (17.9) | 4.41 | 7.90 | 12.80 | |
| Saquinavir (SQV) | 36 (11.1) | 7 (2.2) | 0.45 | 0.00 | 11.53 | |
| Other | Raltegravir (RAL) | 22 (6.8) | 19 (5.9) | 0.05 | 0.00 | 1.90 |
| Dual NRTIs | ZDV + 3TC | 228 (69.7) | 85 (26.0) | 2.93 | 4.59 | 13.07 |
| TDF + FTC | 76 (23.2) | 64 (19.6) | 0.32 | 0.00 | 4.76 | |
| ABC + 3TC | 88 (26.9) | 60 (18.3) | 0.80 | 0.14 | 11.15 | |
| ddI + d4T | 117 (35.8) | 20 (6.1) | 1.51 | 1.88 | 13.30 |
Abbreviations: cART-combination ART, defined as at least 3 drugs from at least two drug classes; NNRTI-non-nucleoside reverse transcriptase inhibitor; NRTI-nucleoside reverse transcriptase inhibitor; PI-protease inhibitor.
Association of Combination Therapy with Cardiac Function and Structure
Current use of cART with a PI at the time of echocardiogram was associated with significantly higher (better) cardiac function as reflected by ejection fraction, contractility, and fractional shortening, with adjusted mean Z-scores which were higher by 0.25 (p=0.03), 0.33 (p=0.01), and 0.28 (p=0.02), respectively, compared to those not receiving PI-based cART. In contrast, the duration of cART use (with or without a PI) was not associated with measures of cardiac function. Neither current use nor duration of cART with a PI was associated with LV structural measures.
Association of Individual Antiretroviral Drugs with Cardiac Function and Structure
After FDR control, models simultaneously evaluating all individual ARVs revealed no significant association for any individual ARV taken at the time of echocardiography with the three functional cardiac measures (Supplemental Table 1). However, youth receiving efavirenz had decreased contractility (adjusted mean difference=−0.40, nominal p=0.04) as did those receiving lamivudine (adjusted mean difference=−0.32, p=0.06). In addition, youth receiving lopinavir had improved cardiac function as measured by ejection fraction and fractional shortening, with Z-scores which were 0.28 and 0.29 higher on average, respectively, compared to those not receiving lopinavir (Table 2). When not accounting for concurrent use of other ARVs, the association of efavirenz with decreased contractility appeared stronger, with a mean decrease of 0.46 (p=0.004), whereas lamivudine showed no association (Table 2); however, as noted previously, these estimates are likely biased given the lack of control for other ARVs in the same regimen. The hierarchical modeling approach yielded results similar to those of the mutually adjusted model. The estimates were slightly attenuated (pulled towards drug class means), but the smaller standard errors reflected increased precision (Table 2). Cumulative duration of use was not associated with any LV function measure for any individual ARV, when adjusting for all ARVs in the current regimen (Supplemental Table 2).
Table 2.
Association of Individual ARVs with LV Function and Structure Measures Based on Separate Models, Mutually Adjusted Models, or Hierarchical Models
| Cardiac Z-score outcome | ARV drug (current use) | Separate Models1 | Mutually Adjusted2 | Hierarchical Model3 | |||
|---|---|---|---|---|---|---|---|
| Est. (se) | P-value | Est. (se) | P-value | Est. (se) | P-value | ||
| Measures of Left Ventricular (LV) Function | |||||||
| Ejection Fraction | Lopinavir (LPV/r) | 0.18 (0.11) | 0.095 | 0.28 (0.14) | 0.052 | 0.28 (0.14) | 0.041 |
| Nelfinavir (NFV) | −0.35 (0.16) | 0.028 | −0.24 (0.21) | 0.25 | −0.19 (0.20) | 0.34 | |
| Ritonavir (RTV) | 0.35 (0.14) | 0.013 | 0.43 (0.25) | 0.088 | 0.40 (0.21) | 0.051 | |
| Contractility | Lamivudine (3TC) | −0.15 (0.13) | 0.24 | −0.32 (0.17) | 0.058 | −0.30 (0.16) | 0.064 |
| Efavirenz (EFV) | −0.46 (0.16) | 0.004* | −0.40 (0.20) | 0.042 | −0.38 (0.19) | 0.048 | |
| Fractional Shortening | Abacavir (ABC) | −0.10 (0.12) | 0.43 | −0.25 (0.14) | 0.075 | −0.23 (0.14) | 0.097 |
| Didanosine (ddI) | 0.31 (0.13) | 0.022 | 0.26 (0.16) | 0.11 | 0.23 (0.15) | 0.14 | |
| Lopinavir (LPV/r) | 0.09 (0.11) | 0.40 | 0.29 (0.15) | 0.056 | 0.29 (0.15) | 0.050 | |
| Ritonavir (RTV) | 0.28 (0.15) | 0.052 | 0.21 (0.25) | 0.40 | 0.26 (0.21) | 0.21 | |
| Measures of Left Ventricular (LV) Structure | |||||||
| LV Mass (2D) | Didanosine (ddI) | −0.25 (0.14) | 0.064 | −0.21 (0.16) | 0.19 | −0.20 (0.16) | 0.19 |
| Efavirenz (EFV) | −0.45 (0.13) | 0.001* | −0.38 (0.17) | 0.023 | −0.37 (0.16) | 0.023 | |
| Zidovudine (ZDV) | 0.21 (0.12) | 0.084 | 0.16 (0.16) | 0.33 | 0.14 (0.16) | 0.33 | |
| LV ED Dimension | Efavirenz (EFV) | −0.32 (0.14) | 0.025 | −0.24 (0.17) | 0.16 | −0.21 (0.18) | 0.16 |
| Lopinavir (LPV/r) | 0.26 (0.12) | 0.025 | 0.17 (0.16) | 0.29 | 0.16 (0.15) | 0.29 | |
| Nevirapine (NVP) | 0.79 (0.25) | 0.002* | 0.70 (0.26) | 0.008 | 0.61 (0.25) | 0.008 | |
| Zidovudine (ZDV) | 0.32 (0.13) | 0.010 | 0.35 (0.17) | 0.036 | 0.32 (0.16) | 0.036 | |
| ES Wall Stress | Amprenavir (APV) | −1.53 (1.08) | 0.16 | −2.19 (1.10) | 0.048 | −0.67 (0.51) | 0.048 |
| Atazanavir (ATV) | −0.38 (0.21) | 0.067 | −0.39 (0.35) | 0.27 | −0.36 (0.27) | 0.27 | |
| Stavudine (d4T) | 0.02 (0.17) | 0.89 | 0.47 (0.23) | 0.040 | 0.37 (0.21) | 0.040 | |
| Efavirenz (EFV) | −0.43 (0.17) | 0.014 | −0.56 (0.21) | 0.007 | −0.52 (0.20) | 0.007 | |
| Ritonavir (RTV) | −0.37 (0.18) | 0.045 | −0.27 (0.34) | 0.43 | −0.29 (0.24) | 0.43 | |
| Tenofovir (TDF) | −0.28 (0.16) | 0.085 | 0.00 (0.25) | 1.00 | 0.01 (0.22) | 1.00 | |
| Zidovudine (ZDV) | 0.46 (0.15) | 0.002* | 0.60 (0.20) | 0.004* | 0.55 (0.19) | 0.004* | |
| LV ES Volume | Lamivudine (3TC) | −0.07 (0.10) | 0.50 | −0.29 (0.15) | 0.047 | −0.26 (0.14) | 0.047 |
| Abacavir (ABC) | 0.14 (0.11) | 0.21 | 0.28 (0.14) | 0.040 | 0.25 (0.13) | 0.040 | |
| Zidovudine (ZDV) | 0.19 (0.11) | 0.095 | 0.25 (0.15) | 0.096 | 0.24 (0.15) | 0.096 | |
| LV ED Volume | Lamivudine (3TC) | −0.10 (0.11) | 0.34 | −0.37 (0.15) | 0.016 | −0.33 (0.14) | 0.016 |
| Efavirenz (EFV) | −0.25 (0.13) | 0.052 | −0.18 (0.16) | 0.28 | −0.18 (0.16) | 0.28 | |
| LV Thickness to Dimension Ratio | Zidovudine (ZDV) | −0.27 (0.11) | 0.012 | −0.37 (0.15) | 0.011 | −0.36 (0.14) | 0.012 |
| ED Septal Thickness | Lamivudine (3TC) | 0.11 (0.10) | 0.25 | 0.27 (0.14) | 0.049 | 0.24 (0.13) | 0.070 |
Only those outcomes and ARVs with nominal p<0.10 by at least one approach are included in Table.
Met criteria for significance after controlling False Discovery Rate (FDR) at 0.10 within each echocardiographic measure
Parameter estimates reflect shift in mean z-score for each ARV drug based on a linear regression model fit separately to each ART medication, adjusting for age and BMI Z-score at echocardiogram, sex, race, and ethnicity.
Parameter estimates refect shift in mean z-score for each ARV drug based on linear regression model including all individual ARVs shown, and adjusting for age and BMI Z-score at echocardiogram, sex, race, and ethnicity.
Parameter estimates reflect shift in mean z-score for each ARV drug based on hierarchical linear model including all individual ARVs shown, nested within their respective drug classes, with fixed effects for drug classes and random effects for individual ARVs.
In evaluating primary and secondary measures of cardiac structure (Supplemental Tables 3 and 4, respectively), adjusted mean Z-scores for LV ED dimension and ES wall stress were higher in youth taking zidovudine at the time of echocardiography than in those not taking zidovudine, with the latter finding meeting the threshold for significance when controlling FDR at the 0.10 level (Table 2). In addition, zidovudine was associated with lower adjusted mean Z-scores for thickness-to-dimension ratio. Current use of stavudine was also associated with higher LV ES wall stress. In contrast, efavirenz was associated with lower LV Mass and lower ES wall stress, and nevirapine was associated with higher LV ED dimension. Few associations were observed for cumulative ARV durations with structural parameters (Supplemental Tables 5 and 6), but longer zidovudine use was associated with higher wall stress (0.05 increase in Z-score per year zidovudine use, p=0.03) and longer raltegravir or saquinavir use was associated with decreased septal wall thickness (0.52 and 0.10 decrease in Z-score per year, p=0.004 and p=0.018, respectively).
The hierarchical model again yielded estimates and conclusions similar to those of the mutually-adjusted model. For example, the estimated mean increase in ES wall stress Z-score with zidovudine exposure was 0.55 (SE=0.19, p=0.004) in the hierarchical model as compared to 0.60 (SE=0.20, p=0.004) in the mutually-adjusted model. However, associations with ARVs rarely used were less often identified than in the mutually-adjusted model; the estimated increase in wall stress with stavudine use was 0.37 (SE=0.21) in the hierarchical model as compared to 0.47 (SE=0.23) in the mutually-adjusted model.
Latent Variable Models for Multiple Measures of Cardiac Function and Structure
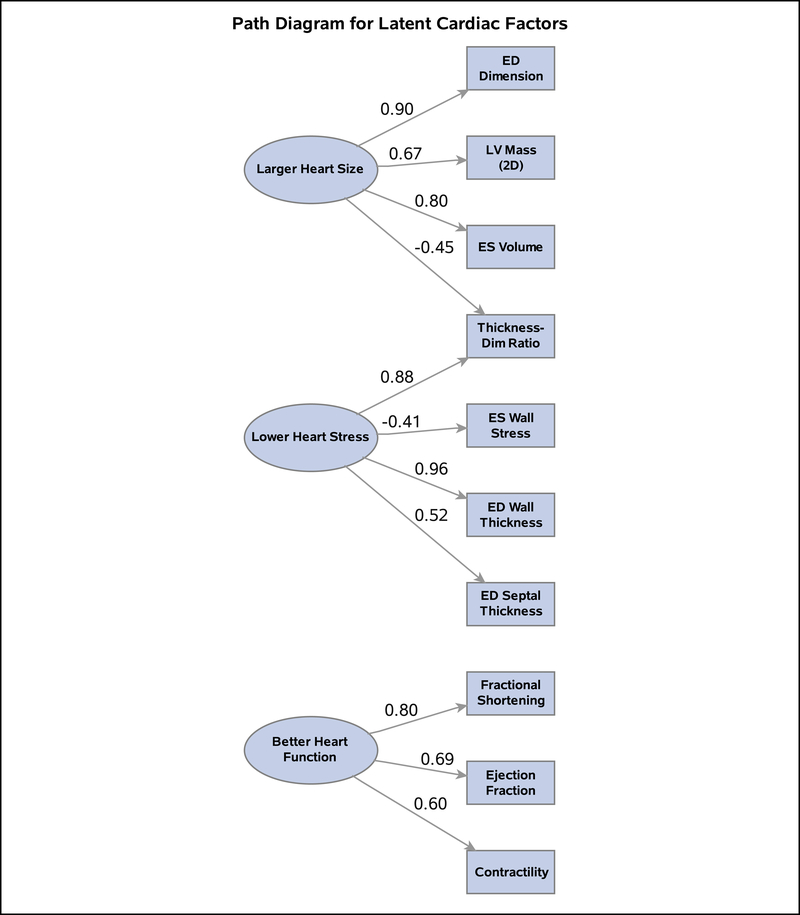
Three factors were identified which explained 71% of total variance, each with clear intuitive meaning (Figure 1). The first factor (“larger heart size”) had high factor loadings on LV mass, ED dimension, and ES volume; the second factor (“lower heart wall stress”) had high positive loadings on LV ED wall thickness, thickness-to-dimension ratio, and ED septal thickness and a negative loading on LV ES wall stress. Lastly, the third factor (“better heart function”) had high positive loadings on contractility, ejection fraction, and fractional shortening. Standardized factor scores (mean=0, SD=1) were obtained for each latent factor, and those for lower heart wall stress were reverse-coded to reflect “higher heart wall stress” for consistency with the adverse direction of larger heart size. Using the standardized heart function factor score as the outcome, lopinavir was associated with improved cardiac function after adjusting for demographic factors and all other ARVs (Table 3). Zidovudine was associated with an adjusted mean increase of 0.32 in heart wall stress factor scores, and both zidovudine and nevirapine were associated with higher adjusted heart size factor scores (by 0.36 and 0.44, respectively) as compared to those not receiving these ARVs.
Figure 1. Path Diagram for Latent Measures of Cardiac Function and Structure.
Latent (underlying) variables for cardiac domains identified in exploratory factor analysis are shown as ovals, while observed echocardiographic measures are shown as rectangles. The standardized loading factors reflect the correlation between the underlying latent variable and the observed echocardiographic measures.
Table 3.
Association of Individual ARVs with Latent Measures of Heart Function, Wall Stress, and Size in Models Mutually Adjusting for All Other ARVs
| Better Heart Function | Larger Heart Size | Higher Heart Wall Stress | ||||
|---|---|---|---|---|---|---|
| ARV Drug or Regimen | Factor Score Est. (se) p-value | Repeated Measures Model Est. (se) p-value | Factor Score Est. (se) p-value | Repeated Measures Model Est. (se) p-value | Factor Score Est. (se) p-value | Repeated Measures Model Est. (se) p-value |
| Model for overall regimen: (cART with PI vs other regimen) | ||||||
| cART with PI | −0.30 (0.11) 0.007 | −0.27 (0.09) 0.003 | 0.10 (0.10) 0.35 | 0.10 (0.09) 0.30 | 0.03 (0.12) 0.79 | −0.004 (0.09) 0.96 |
| Mutually adjusted models including all ARVs | ||||||
| ART Drug Class: NRTIs | ||||||
| Lamivudine (3TC) | −0.02 (0.14) 0.89 | −0.07 (0.12) 0.58 | −0.18 (0.13) 0.18 | −0.21 (0.12) 0.081 | −0.02 (0.16) 0.91 | −0.16 (0.11) 0.17 |
| Abacavir (ABC) | −0.25 (0.14) 0.067 | −0.16 (0.11) 0.15 | 0.19 (0.12) 0.13 | 0.12 (0.12) 0.30 | 0.09 (0.15) 0.55 | 0.13 (0.11) 0.22 |
| Zidovudine (ZDV) | −0.07 (0.15) 0.64 | −0.05 (0.13) 0.68 | 0.36 (0.14) 0.010 | 0.23 (0.13) 0.079 | 0.32 (0.17) 0.061 | 0.31 (0.12) 0.011 |
| ART Drug Class: NNRTIs | ||||||
| Efavirenz (EFV) | 0.02 (0.16) 0.92 | −0.06 (0.13) 0.67 | −0.17 (0.14) 0.23 | −0.26 (0.13) 0.054 | −0.07 (0.17) 0.67 | −0.08 (0.12) 0.50 |
| Nevirapine (NVP) | 0.05 (0.24) 0.84 | −0.13 (0.19) 0.50 | 0.44 (0.21) 0.038 | 0.26 (0.20) 0.19 | −0.04 (0.26) 0.88 | −0.03 (0.19) 0.89 |
| ART Drug Class: PIs | ||||||
| Lopinavir (LPV/r) | 0.31 (0.15) 0.034 | 0.21 (0.12) 0.084 | 0.16 (0.13) 0.23 | 0.08 (0.12) 0.50 | 0.02 (0.16) 0.88 | −0.01 (0.11) 0.90 |
cART=combination antiretroviral therapy; NNRTI-non-nucleoside reverse transcriptase inhibitor; NRTI-nucleoside reverse transcriptase inhibitor; PI=protease inhibitor; se=standard error
All models adjust for all other ARV drugs, along with age, sex, race, ethnicity, and BMI Z-score at echocardiogram.
Parameter estimates reflect shift in mean z-score for each ARV drug. Only those ARV drugs with p<0.10 for at least one latent outcome are shown.
Repeated measures models include correlated set of echocardiogram parameters assuming unstructured covariance:
for Function: ejection fraction, fractional shortening, and contractility; for Size: LV mass, ED Dimension, ES volume; for Stress: ES Wall Stress, Thickness-to-Dimension Ratio, ED Wall Thickness, ED Septal Thickness.
The repeated measures models for correlated vectors of echocardiographic measures yielded findings similar to the models of a single factor score within each domain. However, despite the fact that the repeated measures models each considered multiple echocardiographic outcomes simultaneously and accounted for their inter-correlation, standard errors were consistently smaller for estimated associations than when modeling each single factor score (Table 3). Evaluating cART with a PI as an overall regimen using the factor scores and a repeated measures model for latent variables indicated a significant association with higher heart function but no association with heart size or wall stress, consistent with findings of individual echocardiographic parameters.
Sensitivity Analyses Accounting for HIV Disease Severity
Sensitivity analyses further adjusting the mutually adjusted ARV models for unsuppressed VL, low nadir CD4%, and CDC Class C diagnosis yielded almost identical findings, suggesting little evidence of confounding by indication. For example, the estimated increase in wall stress with zidovudine was 0.59 (SE=0.21, p=0.004) after adjusting for VL greater than 5000 cp/mL, as compared to 0.60 before such adjustment; the mean decrease in LV mass with efavirenz exposure was 0.39 (SE=0.17) with additional adjustment for unsuppressed VL and 0.38 (SE=0.17) without adjustment (data not shown).
DISCUSSION
Combination ART regimens have been linked to improved cardiac health in children with PHIV, with overt symptoms of cardiomyopathy rarely observed in the current era [1–7,12]. Despite this success, individual ARVs could still cause cardiotoxicities, and given the wide range of choices in combination regimens, identifying those with the safest toxicity profile is warranted. However, evaluating individual ARVs is complicated by their concurrent use with other ARVs in combination regimens [30]. We used novel statistical techniques to examine associations of individual ARVs with cardiac function and structure, accounting for other ARVs taken concurrently and for multiple statistical comparisons resulting from analyzing multiple echocardiographic measures.
Some individual ARVs showed consistent patterns of associations with separate echocardiographic measures of cardiac structure, specifically, a larger heart size (LV mass, volume, and/or ED dimension) with use of zidovudine and nevirapine, smaller heart size with efavirenz, and increased LV ES wall stress with zidovudine. Only the association of zidovudine with increased ES wall stress was statistically significant after FDR correction, but the overall pattern across multiple structural measures provided additional support for an association. Fewer associations were observed with separate echocardiographic measures of cardiac function, although youth taking lopinavir had higher LV ejection fraction and fractional shortening and efavirenz was linked to lower LV contractility before correcting for multiple comparisons. The apparent benefit of lopinavir on LV function was unexpected, and not consistent with adverse associations of PI-based regimens on cardiac health noted in adults [13–15].
Use of latent variable models for underlying measures of heart function, size, and wall stress reinforced the findings of separate echocardiographic parameters, indicating an adverse association of zidovudine with both larger heart size and higher heart wall stress and a beneficial association of lopinavir with higher heart function. While shifts in mean z-scores for these ARV drugs were relatively small, even small increases may have implications at the population level in terms of increased prevalence of clinical conditions such as left ventricular hypertrophy or cardiomyopathy. The subclinical differences in cardiac structure observed in this age group could portend future adverse cardiac outcomes as these children become adults and other cardiometabolic risk factors such as obesity, smoking, hypertension and hypercholesterolemia become more common [9,10,31]. Many of these risk factors are more prevalent in youth with HIV than similarly-aged children in the general population [32–35], and PIs, as a drug class, have often been implicated in metabolic risk factors for cardiovascular disease. However, our results more often identified adverse effects with NRTIs and NNRTIs, which may act through other mechanisms such as mitochondrial toxicity. Lopinavir appeared to be protective and no other PIs were associated with adverse function or increased heart wall stress or size.
Our observational study of individual ARV effects has certain limitations. We cannot rule out residual confounding, including possible confounding by indication which could result if children with more severe disease were more likely to be treated with certain medications. Sensitivity analyses controlling for measures of both current and past HIV disease severity produced almost identical results, which suggests that the effect of such confounding is limited. In addition, in the current era, HIV disease severity has not been associated with cardiac structure or function among otherwise healthy and virologically-suppressed youth [7]. We used several approaches to limit false positives, but because we are concerned with the safety profile of ARVs, we must also consider Type II errors (missing true associations). We did not include ARVs with very rare exposures, including several newer medications, such as dolutegravir, and ones no longer used, such as indinavir and zalcitabine. Continued monitoring is clearly warranted, particularly as newer agents become prescribed more widely.
The strengths of our study include the use of several novel statistical techniques that allowed us to evaluate a large number of individual ARVs and to reduce multiple cardiac outcomes to manageable number. In addition, our hierarchical approach took advantage of the fact that mechanisms of action are often similar within a single drug class [36–38]. In this particular evaluation, the hierarchical model provided results similar to those of the mutually adjusted model and offered little additional benefit, but the hierarchical modeling strategy can provide a distinct advantage when considering binary outcomes by ensuring convergence despite high dimensional exposures [23]. Ideally, a comparative safety evaluation would subject a small number of specific cART regimens in a head-to-head comparison [39–41]. However, this approach was not feasible here given that 137 different cART regimens were reported at the time of echocardiography, with the 5 most common regimens representing only 26% of the total. Nevertheless, further research is warranted for specific combinations of ARVs including fixed dose combinations, as well as dual NRTI backbones which continue to play a central role in HIV treatment strategies but have been linked to toxicity across multiple organ systems [42,43]. The analytic approach described here could be used by investigators evaluating the relationships between large numbers of different ARVs and combination regimens with adverse outcomes in other organ systems.
In conclusion, we observed beneficial effects of cART regimens which included protease inhibitors on cardiac outcomes in youth with PHIV. However, given the large number of options for specific ARV drugs that can be included in combination regimens, some ARVs such as zidovudine could be replaced by those with more favorable toxicity profiles. Longitudinal studies are needed to evaluate longer-term trends and early cardiovascular risk factors among youth with HIV to develop targeted interventions for those at highest risk of cardiovascular disease.
Supplementary Material
Acknowledgements
We thank the children and families for their participation in PHACS, and the individuals and institutions involved in the conduct of PHACS. The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard T.H. Chan School of Public Health (HD052102) (Principal Investigator: George Seage; Project Director: Julie Alperen) and the Tulane University School of Medicine (HD052104) (Principal Investigator: Russell Van Dyke; Co-Principal Investigator: Ellen Chadwick; Project Director: Patrick Davis). Data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc (PI: Julie Davidson).
The following institutions, clinical site investigators and staff participated in conducting PHACS AMP and AMP Up in 2016, in alphabetical order: Ann & Robert H. Lurie Children’s Hospital of Chicago: Ram Yogev, Margaret Ann Sanders, Kathleen Malee, Scott Hunter; Baylor College of Medicine: William Shearer, Mary Paul, Norma Cooper, Lynnette Harris; Bronx Lebanon Hospital Center: Murli Purswani, Mahboobullah Mirza Baig, Alma Villegas; Children’s Diagnostic & Treatment Center: Ana Puga, Sandra Navarro, Patricia A. Garvie, James Blood; Boston Children’s Hospital: Sandra K. Burchett, Nancy Karthas, Betsy Kammerer; Jacobi Medical Center: Andrew Wiznia, Marlene Burey, Ray Shaw, Raphaelle Auguste; Rutgers - New Jersey Medical School: Arry Dieudonne, Linda Bettica, Juliette Johnson; St. Christopher’s Hospital for Children: Janet S. Chen, Maria Garcia Bulkley, Latreaca Ivey, Mitzie Grant; St. Jude Children’s Research Hospital: Katherine Knapp, Kim Allison, Megan Wilkins, Jamie Russell-Bell; San Juan Hospital/Department of Pediatrics: Midnela Acevedo-Flores, Heida Rios, Vivian Olivera; Tulane University School of Medicine: Margarita Silio, Medea Gabriel, Patricia Sirois; University of California, San Diego: Stephen A. Spector, Kim Norris, Sharon Nichols; University of Colorado Denver Health Sciences Center: Elizabeth McFarland, Eric Cagwin, Emily Barr, Alisa Katai; University of Miami: Gwendolyn Scott, Grace Alvarez, Gabriel Fernandez, Anai Cuadra.
Note: The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or U.S. Department of Health and Human Services.
Sources of Funding:
The Pediatric HIV/AIDS Cohort Study (PHACS) was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) with co-funding from the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute of Allergy and Infectious Diseases (NIAID), the National Institute of Neurological Disorders and Stroke (NINDS), the National Institute on Deafness and Other Communication Disorders (NIDCD), Office of AIDS Research (OAR), the National Institute of Mental Health (NIMH), the National Institute on Drug Abuse (NIDA), and the National Institute on Alcohol Abuse and Alcoholism (NIAAA), through cooperative agreements with the Harvard T.H. Chan School of Public Health (HD052102) and the Tulane University School of Medicine (HD052104).
Footnotes
Conflicts of Interest: None reported.
REFERENCES
- 1.Luginbuhl LM, Orav EJ, McIntosh K, Lipshultz SE. Cardiac morbidity and related mortality in children with HIV infection. JAMA. 1993;269(22):2869–2875. [PubMed] [Google Scholar]
- 2.Lipshultz SE, Easley KA, Orav EJ, et al. Cardiac dysfunction and mortality in HIV-infected children: the prospective P2C2 HIV multicenter study. Pediatric Pulmonary and Cardiac Complications of Vertically Transmitted HIV Infection (P2C2 HIV) Study Group. Circulation. 2000;102(13):1542–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langston C, Cooper ER, Goldfarb J, et al. Human immunodeficiency virus-related mortality in infants and children: data from the pediatric pulmonary and cardiovascular complications of vertically transmitted HIV (P2C2) study. Pediatrics. 2001;107(2):328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher SD, Easley KA, Orav EJ, et al. Mild dilated cardiomyopathy and increased left ventricular mass predict mortality: the prospective P2C2 HIV multicenter study. Am Heart J. 2005; 150(3):439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel K, Van Dyke RB, Mittleman MA, Colan SD, Oleske JM, Seage GR 3rd; International Maternal Pediatric Adolescent AIDS Clinical Trials 219/219C Study Team. The impact of HAART on cardiomyopathy among children and adolescents perinatally infected with HIV-1. AIDS. 2012; 26(16):2027–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipshultz SE, Miller TL, Wilkinson JD, et al. Cardiac effects in perinatally HIV-infected and HIV-exposed but uninfected children and adolescents: a view from the United States of America. J Int AIDS Soc 2013; 16:18597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipshultz SE, Williams PL, Wilkinson JD, et al. ; Pediatric HIV/AIDS Cohort Study (PHACS). Cardiac status of children infected with human immunodeficiency virus who are receiving long-term combination antiretroviral therapy: results from the Adolescent Master Protocol of the Multicenter Pediatric HIV/AIDS Cohort Study. JAMA Pediatr 2013; 167(6):520–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lumsden RH, Bloomfield GS. The causes of HIV-associated cardiomyopathy: a tale of two worlds. BioMed Res Int 2016; 8196560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doom JR, Mason SM, Suglia SF, Clark CJ. Pathways between childhood/adolescent adversity, adolescent socioeconomic status, and long-term cardiovascular disease risk in young adulthood. Soc Sci Med. 2017; 188:166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark CJ, Alonso A, Spencer RA, Pencina M, Williams K, Everson-Rose SA. Predicted long-term cardiovascular risk among young adults in the national longitudinal study of adolescent health. Am J Public Health. 2014; 104(12):e108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipshultz SE, Easley KA, Orav EJ, et al. Left ventricular structure and function in children infected with human immunodeficiency virus: the prospective P2C2 HIV Multicenter Study. Pediatric Pulmonary and Cardiac Complications of Vertically Transmitted HIV Infection (P2C2 HIV) Study Group. Circulation 1998;97(13):1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipshultz SE, Wilkinson JD, Thompson B, et al. for the CHAART 2 Investigator Group. Cardiac effects of highly active antiretroviral therapy in perinatally HIV-infected children: The CHAART 2 Study. J Am Coll Cardiol 2017; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Worm SW, Sabin C, Weber R, Reiss P, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis. 2010;201(3):318–30. [DOI] [PubMed] [Google Scholar]
- 14.Holmberg SD, Moorman AC, Williamson JM, et al. ; HIV Outpatient Study (HOPS) investigators. Protease inhibitors and cardiovascular outcomes in patients with HIV-1. Lancet. 2002; 360(9347):1747–8. [DOI] [PubMed] [Google Scholar]
- 15.Sabin CA, Reiss P, Ryom L, et al. ; D:A:D Study Group. Is there continued evidence for an association between abacavir usage and myocardial infarction risk in individuals with HIV? A cohort collaboration. BMC Med. 2016;14:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sibiude J, Le Chenadec J, Bonnet D, et al. ; French National Agency for Research on AIDS and Viral Hepatitis French Perinatal Cohort/Protease Inhibitor Monotherapy Evaluation Trial. In utero exposure to zidovudine and heart anomalies in the ANRS French perinatal cohort and the nested PRIMEVA randomized trial. Clin Infect Dis. 2015. July 15;61(2):270–80. [DOI] [PubMed] [Google Scholar]
- 17.Poirier MC, Gibbons AT, Rugeles MT, Andre-Schmutz I, Blanche S. Fetal consequences of maternal antiretroviral nucleoside reverse transcriptase inhibitor use in human and nonhuman primate pregnancy. Curr Opin Pediatr. 015 April;27(2):233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.García-Otero L, López M, Gómez O, et al. Zidovudine treatment in HIV-infected pregnant women is associated with fetal cardiac remodelling. AIDS. 2016. June 1;30(9):1393–401. [DOI] [PubMed] [Google Scholar]
- 19.Rough K, Sun JW, Seage GR 3rd, et al. Zidovudine use in pregnancy and congenital malformations. AIDS. 2017;31(12):1733–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torres SM, March TH, Carter MM, et al. In utero exposure of female CD-1 mice to AZT and/or 3TC: I. Persistence of microscopic lesions in cardiac tissue. Cardiovasc Toxicol. 2010;10(1):37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torres SM, Divi RL, Walker DM, et al. In utero exposure of female CD-1 mice to AZT and/or 3TC: II. Persistence of functional alterations in cardiac tissue. Cardiovasc Toxicol. 2010;10(2):87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blanche S, Tardieu M, Rustin P, et al. Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet. 1999;354(9184):1084–1089. [DOI] [PubMed] [Google Scholar]
- 23.Correia K, Williams PL. A hierarchical modeling approach for assessing the safety of exposure to complex antiretroviral drug regimens during pregnancy. Stat Methods Med Res 2017; 962280217732597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. 2005;99(2):445–457. [DOI] [PubMed] [Google Scholar]
- 25.Benjamini Y, Hochberg Y Controlling the False Discovery Rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Ser B 1995;57(1):289–300. [Google Scholar]
- 26.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001; 125(1–2):279–84. [DOI] [PubMed] [Google Scholar]
- 27.Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data via the EM algorithm. Journal Royal Statistical Society, Series B 1977; 39:1–38. [Google Scholar]
- 28.Verbeke G, Molenbergs G. Linear Mixed Models for Longitudinal Data. 2000; Springer-Verlag: New York. [Google Scholar]
- 29.Christensen R, Pearson LM, and Johnson W Case-deletion Diagnostics for Mixed Models. Technometrics 1992; 34, 38–45 [Google Scholar]
- 30.Zash RM, Williams PL, Sibiude J, Lyall H, Kakkar F. Surveillance monitoring for safety of in utero antiretroviral therapy exposures: current strategies and challenges. Expert Opin Drug Saf. 2016;15(11):1501–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipshultz SE, Fisher SD, Lai WW, Miller TL. Cardiovascular risk factors, monitoring, and therapy for HIV-infected patients. AIDS. 2003;17 Suppl 1:S96–122. [DOI] [PubMed] [Google Scholar]
- 32.Patel K, Wang J, Jacobson DL, et al. ; Pediatric HIV/AIDS Cohort Study (PHACS). Aggregate risk of cardiovascular disease among adolescents perinatally infected with the human immunodeficiency virus. Circulation. 2014;129(11):1204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCrindle BW, Urbina EM, Dennison BA, et al. ; American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee; American Heart Association Council of Cardiovascular Disease in the Young; American Heart Association Council on Cardiovascular Nursing. Drug therapy of high-risk lipid abnormalities in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee, Council of Cardiovascular Disease in the Young, with the Council on Cardiovascular Nursing. Circulation. 2007;115(14):1948–67. [DOI] [PubMed] [Google Scholar]
- 34.Farley J, Gona P, Crain M, et al. ; Pediatric AIDS Clinical Trials Group Study 219C Team. Prevalence of elevated cholesterol and associated risk factors among perinatally HIV-infected children (4–19 years old) in Pediatric AIDS Clinical Trials Group 219C. J Acquir Immune Defic Syndr. 2005;38(4):480–7. [DOI] [PubMed] [Google Scholar]
- 35.Tassiopoulos K, Williams PL, Seage GR 3rd, Crain M, Oleske J, Farley J; International Maternal Pediatric Adolescent AIDS Clinical Trials 219C Team. Association of hypercholesterolemia incidence with antiretroviral treatment, including protease inhibitors, among perinatally HIV-infected children. J Acquir Immune Defic Syndr. 2008;47(5):607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cihlar T and Ray A. Nucleoside and nucleotide HIV reverse transcriptase inhibitors: 25 years after Zidovudine. Antiviral Res 2010; 85: 39–58. [DOI] [PubMed] [Google Scholar]
- 37.De Bethune MP. Non-nucleoside reverse transcriptase inhibitors (NNRTIs), their discovery, development, and use in the treatment of HIV-1 infection: a review of the last 20 years (1989–2009). Antiviral Res 2010; 85: 75–90. [DOI] [PubMed] [Google Scholar]
- 38.Wensing AMJ, van Maarseveen NM and Nijhuis M. Fifteen years of HIV protease inhibitors: raising the barrier to resistance. Antiviral Res 2010; 85: 59–74. [DOI] [PubMed] [Google Scholar]
- 39.Kanters S, Socias ME, Paton NI, et al. Comparative efficacy and safety of second-line antiretroviral therapy for treatment of HIV/AIDS: a systematic review and network meta-analysis. Lancet HIV 2017; 4(10):e433–441. [DOI] [PubMed] [Google Scholar]
- 40.Lodi S, Phillips A, Logan R, et al. ; HIV-CAUSAL Collaboration. Comparative effectiveness of immediate antiretroviral therapy versus CD4-based initiation in HIV-positive individuals in high-income countries: observational cohort study. Lancet HIV. 2015;2(8):e335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caniglia EC, Patel K, Huo Y, et al. ; Pediatric HIVAIDS Cohort Study. Atazanavir exposure in utero and neurodevelopment in infants: a comparative safety study. AIDS. 2016;30(8):1267–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lipshultz SE, Shearer WT, Thompson B, et al. Antiretroviral therapy (ART) cardiac effects in HIV-infected children: the multicenter NHLBI Cardiac Highly Active Antiretroviral Therapy (CHAART-II) study. Circulation. 2009;120:S909–S910. [Google Scholar]
- 43.Van Dyke RB, Wang L, Williams PL; Pediatric AIDS Clinical Trials Group 219C Team. Toxicities associated with dual nucleoside reverse-transcriptase inhibitor regimens in HIV-infected children. J Infect Dis. 2008;198(11):1599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.