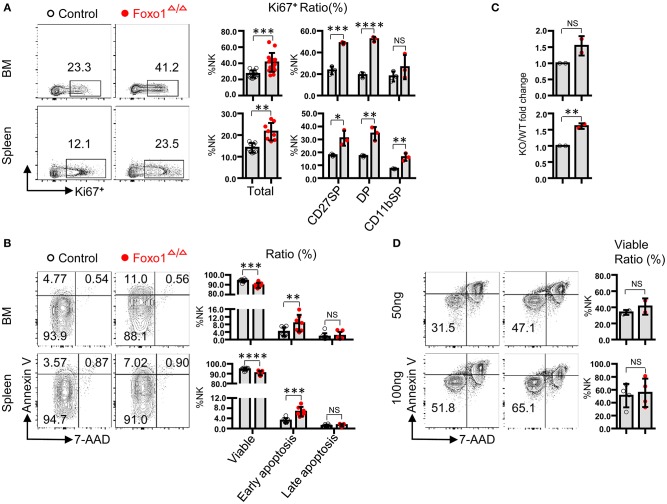
Figure 2.
Hematopoietic-specific deletion of Foxo1 leads to increased proliferation of NK cells. (A) Intracellular flow cytometric analysis of Ki-67+ cells in total NK cells (CD3−CD19− NKp46+) and within indicated subpopulations in both the BM and spleen from control vs. Foxo1Δ/Δ mice. (B) Flow cytometric analysis of the apoptosis of NK cells (CD3−CD19− NKp46+) in both the BM and spleen from control vs. Foxo1Δ/Δ mice. Viable: Annexin V−7-AAD−subpopulation; early apoptosis: Annexin V+7-AAD−subpopulation; late apoptosis: Annexin V+7-AAD+subpopulation. (C,D) Enumeration (C) and Flow cytometric analysis of apoptosis (D) of sorted NK cells (CD3−CD19− NKp46+) after in vitro stimulation with 50 or 100 ng/ml IL-15 for 5 days from control vs. Foxo1Δ/Δ mice. Each dot represents one mouse. Four and six littermates were included for (A,B), respectively; 2 littermates were included for (C) 2 and 4 littermates were included for 50 and 100 ng/ml IL-15 stimulation, respectively, for (D). (Error bars indicate SD; unpaired Student's t-test with generalized linear models; *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001, control vs. Foxo1Δ/Δ mice).