Figure 1.
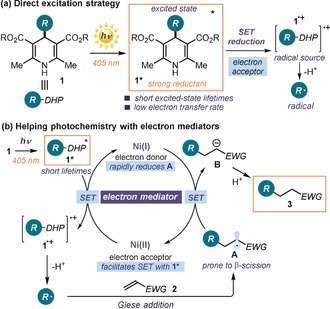
(a) The excited‐state reactivity of 4‐alkyl‐1,4‐dihydropyridines (R‐DHPs, 1): on excitation, they become both photoreductants and precursors of alkyl radicals. (b) Proposed strategy to realize the Giese reaction by combining the photochemistry of 1 and the action of a catalytic nickel complex, which facilitates the redox processes by acting as an electron mediator (EM); NiII rapidly oxidizes the short‐lived, excited R‐DHPs 1* while the ensuing NiI species reduces the highly reactive intermediate A, which has a high tendency to undergo side reactions. The overall sequence affords product 3.
