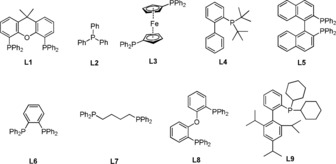
Table 3.
Screen of ligand for the isomerization of 4‐phenyl‐1‐butene.[a]
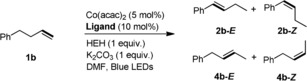
| Entry | Ligand | Conv. [%] | 2 b [%][b] (E/Z)[c] | 4 b [%][b] (E/Z)[c] |
|---|---|---|---|---|
| 1 | L1 | 100 | 93 (98/2) | 4 |
| 2 | L2 | 57 | 18 (98/2) | 36 (78/22) |
| 3 | L3 | 0 | 0 | 0 |
| 4 | L4 | 2 | 0 | 0 |
| 5 | L5 | 10 | 2 | 4 |
| 6 | L6 | 4 | 0 | 0 |
| 7 | L7 | 90 | 47 (99/1) | 37 (64/36) |
| 8 | L8 | 98 | 5 | 90 (80/20) |
| 9 | L9 | 16 | 2 | 3 |
| 10[d] | L8 | 94 | 0 | 89 (70/30) |
| 11[e] | L8 | 81 | 0 | 78 (59/41) |
[a] Unless otherwise noted, all the reactions were carried out with 4‐phenyl‐1‐butene (0.2 mmol), Co(acac)2 (0.01 mmol), ligand (0.02 mmol), HEH (0.2 mmol), and K2CO3 (0.2 mmol) in DMF (2 mL) under N2, irradiation with blue LEDs at 25 °C for 16 hours. [b] GC‐FID yield using 1,3,5‐trimethoxybenzene as an internal standard. [c] The ratio of E/Z was determined by 1H NMR. [d] 0.1 mmol HEH was used. [e] 0.04 mmol HEH was used.