Abstract
Increased error-related negativity (ERN) has been implicated in the pathophysiology of multiple forms of psychopathology. Although there is increasing evidence that the ERN can be shaped by environment and experience, no studies to date have examined this question in a clinical sample. In the current study, we examined the influence of combat exposure on the ERN using electroencephalogram (EEG) in a sample of military veterans with a high prevalence of psychopathology. Participants included sixty-seven U.S. military veterans from Operations Enduring Freedom, Iraqi Freedom, and New Dawn (OEF/OIF/OND). The degree of combat exposure was assessed using the Deployment Risk and Resilience Inventory-2 (DRRI-2) and Combat Exposure Scale (CES). A well-validated flanker task was used to elicit the ERN during continuous EEG recording. Results revealed that veterans who reported experiencing greater combat exposure exhibited a more enhanced ERN, even when adjusting for broad anxiety and posttraumatic stress disorder (PTSD) symptoms. The association between combat exposure and ERN was not moderated by PTSD symptom severity. The current study demonstrates that greater combat exposure is associated with a more enhanced ERN among OEF/OIF/OND veterans. This enhanced ERN may be one mechanism that places veterans at greater risk for developing psychiatric disorders following exposure to combat. Future longitudinal studies are needed to directly test whether the ERN mediates the relation between level of combat exposure and the development of internalizing disorders.
Keywords: combat exposure, error monitoring, PTSD, error-related negativity, veterans, event-related potentials
1. Introduction
Increased neural response to errors has been implicated in the pathophysiology of multiple forms of psychopathology, including anxiety disorders and alcohol use disorders (Gorka et al., 2016; Gorka et al., 2017; Weinberg, Riesel, & Hajcak, 2012). To capture these effects at the psychophysiological level, researchers have utilized the error-related negativity (ERN), a negative-going deflection in the event-related potential (ERP) waveform that occurs approximately 50–100ms following the commission of an error (e.g., Hajcak & Foti, 2008). The ERN is thought to be generated by the anterior cingulate cortex (ACC), a region associated with responding to negative emotional stimuli and cognitive conflict (Milter et al., 2003). Consistent with the notion that the ERN is a measure of defensive reactivity and threat responding, the ERN amplitude is positively associated with other indices of threat reactivity such as the magnitude of the startle reflex following the commission of an error (Meyer et al., 2017).
Recent research suggests that negative environmental experiences can shape neural response to errors (e.g., Endrass et al., 2010; Meyer & Gawlowska, 2017; Riesel, Weinberg, Endrass, Kathmann, & Hajcak, 2012), albeit with mixed evidence (Moser et al., 2005). For example, the ERN has been shown to be potentiated when errors are made among highly anxious individuals, relative to less anxious individuals (Meyer & Gawlowska, 2017). In a separate study, larger increases in negative affect following a negative mood induction were associated with a more enhanced ERN (Olvet & Hajcak, 2011). However, not all studies have found that inducing negative affect potentiates the ERN, especially when the induction does not involve making mistakes. For instance, one study showed that adults with spider phobia did not exhibit a change in the ERN after being exposed to a tarantula while performing the task (Moser et al., 2005). A limitation to the majority of previous studies examining the relation between environmental experiences and ERN is the examination of negative experiences in the laboratory (i.e., exposure to negative images, scenes, and events) versus capturing exposure to stressful or threatening stimuli in the natural environment. Although no studies to date have examined whether exposure to negative experiences (i.e., trauma) influences the ERN, there is evidence from neuroimaging studies that trauma history can influence alterations in structure, function, and connectivity of several neural regions involved in cognitive and emotional processing, including the ACC (for a review, see Thomason & Marusak, 2017), the region in which the ERN is thought to be localized (Milter et al., 2003).
One population experiencing a high degree of trauma and stress is military service members exposed to combat during deployment. Due to the inherently dangerous nature of a war-zone, veterans in combat might be particularly reactive to the commission of an error, as these errors may threaten their safety. For example, a soldier may die by accidentally stepping on an improvised explosive device (IED), or may harm a fellow soldier by misfiring weapons. Veterans may therefore develop a learned sensitivity to the commission of errors, which could increase the ERN and facilitate survival during combat. However, this enhanced ERN may become problematic in the long term by making veterans exhibit overactive performance monitoring and excessive worry outside of dangerous environments. Thus, an enhanced ERN response might be one mechanism implicated in the high documented rates of psychopathology among veterans returning from combat (Hoge, Auchterlonie, & Milliken, 2006).
Although no studies to date have directly examined whether the degree of combat exposure is associated with the magnitude of the ERN, researchers have compared ERN amplitude between veterans and healthy controls participants. For examples, studies have failed to find a significant difference in the magnitude of ERN when comparing healthy controls to veterans with PTSD (Rabinak et al., 2013; Swick, Honzel, & Turken, 2015). However, one study showed that combat-exposed veterans with no history of psychopathology exhibited a smaller ERN relative to healthy controls and combat-exposed veterans with PTSD (Rabinak et al., 2013). Notably, none of these prior studies examined how individual differences in the degree of combat exposure may influence ERN amplitude. However, there is evidence from behavioral studies to suggest that attention to threat is directly impacted by the amount of exposure to war-related stressors in the laboratory (Bar-Haim et al., 2010).
In the current study, we therefore sought to examine the influence of combat exposure on ERN in a sample of military veterans post-deployment to Iraq or Afghanistan during Operations Enduring Freedom, Iraqi Freedom, and New Dawn (OEF/OIF/OND). To assess combat experiences, we utilized the widely-validated Deployment Risk and Resilience Inventory-2 (DRRI-2; Vogt et al., 2013) and Combat Exposure Scale (CES; Keane et al., 1989). Both of these measures include a series of questions regarding combat experiences (deployment factors), such as witnessing of civilians or combatants being killed or seriously injured, being exposed to fires, and other combat-related events. Consistent with the notion that ERN can be influenced by environmental experiences (Olvet & Hajcak, 2011; Meyer & Gawlowska, 2017; but see Moser et al., 2005), we predicted that there would be a relationship between the ERN and combat exposure, such that veterans with a greater number of combat experiences would exhibit a more enhanced ERN. Finally, to explore whether the current effects were specific to the degree of combat exposure (versus other forms of life stress), we also examined whether ERN amplitude was related to pre- and post-deployment stressors.
2. Methods
2.1. Participants
Sixty-seven participants were recruited from the Jesse Brown VA Medical Center with a wide range of PTSD symptoms. All participants were screened using M.I.N.I. (Mini International Neuropsychiatric Interview) to assess for psychiatric diagnoses and CAPS (Clinician-Administered PTSD Scale, CAPS-IV) to assess for PTSD symptoms. Table 1 displays a summary of the diagnostic data among the participants in the study. Exclusionary criteria in the current study included a history of schizophrenia, clinically significant neurological or medical condition, and alcohol or drug use that would interfere with completion of the study protocol.
Table 1.
Characteristics of sample.
| Mean (SD) | |
|---|---|
| Age | 32.75 (5.80) |
| CAPS | 43.89 (29.40) |
| BAI | 16.02 (12.94) |
| DRRI-2-CE | 36.55 (19.43) |
| CES | 16.27 (9.66) |
| ERN-Error (uV) | 1.05 (6.26) |
| ERN-Correct (uV) | 6.40 (5.10) |
| Error RT (ms) | 335.70 (79.55) |
| Correct RT (ms) | 428.43 (107.46) |
| Accuracy | .89 (.11) |
| N (%) | |
| Sex (Male) | 55 (82.10) |
| Current PTSD | 32 (47.80) |
| Current MDD | 16 (23.90) |
| Current OCD | 4 (6.0) |
| Current Panic Disorder | 14 (20.90) |
| Current GAD | 4 (6.0) |
| Current Social Anxiety | 5 (7.50) |
| Current Alcohol Abuse | 8 (11.90) |
| Current Alcohol Dependence | 15 (22.4) |
| Current Substance Abuse | 3 (4.50) |
| Current Substance Dependence | 10 (14.19) |
Note: CAPS = Clinician-Administered PTSD Scale; BAI = Beck Anxiety Inventory; DRRI-2-CE = Deployment Risk and Resiliency Inventory – Combat Experiences Scale; CES = Combat Exposure Scale; ERN = Error Related Negativity; RT = Response Time; PTSD = Post Traumatic Stress Disorder; OCD = Obsessive Compulsive Disorder; GAD = Generalized Anxiety Disorder; MDD = Major Depressive Disorder.
2.2. Measures
Clinical Measures.
Diagnostic criteria were assessed according to Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) and using the Mini-Interactional Neuropsychiatric Interview (MINI; Sheehan et al., 1998). The Clinician-Administered PTSD Scale (CAPS; Blake et al., 1995) was administered to all participants to determine PTSD symptom severity. Participants also completed the well-validated Beck Anxiety Inventory (BAI; Beck & Steer, 1990) to assess for symptoms of anxiety over the past week.
Combat Exposure and Stress.
Participants were administered the DRRI-2 (Deployment Risk and Resilience Inventory-2; Vogt et al., 2013), a questionnaire with high validity and reliability designed to assess various aspects relating to deployment. The DRRI-2 includes 17 scales measuring pre-deployment (prior stressors and family functioning during childhood), deployment (including combat experiences and perceived threat) and post-deployment factors (stressors, social support, and family functioning). Each section comprises of a series of questions for which the participants rank their responses. Given the aims of the study, we were particularly interested in Section D (Combat Experiences). Items (a total of 17) from Section D included “I was exposed to incoming fire” and “I personally witnessed civilians…being seriously wounded or killed.” Responses ranged on a scale of 1 being the lowest (“Never”) to 6 being the highest (“Daily or almost daily”). The scores from the 17 items were summed for each participant. To assess other forms of life stressors not specific to combat, we included Section A (Predeployment Life Events, 18 items) and Section N (Postdeployment Life Events, 14 items). The items from the subscales assess whether or not (Yes or No) participants experienced a particular stressful event (e.g., financial issues, being robbed or attacked, physically abused, etc.). Items for which participants answered “yes” to were summed for a total score.
Participants also completed the Combat Exposure Scale (CES; Keane et al., 1989). The CES is a 7-item self-report measure of wartime stressors. Responses ranged on a scale of 1 being the lowest (“Never”) to 5 being the highest (“more than 50 times”). The scores from the 5 items were summed for each participant.
Error Monitoring Task.
Participants were seated at a viewing distance of approximately 24 in. (61 cm) from the computer monitor and were told to complete a flanker task that was administered on a PentiumD class computer with a 19-in. (48.3 cm) monitor using Presentation software. During each trial, participants viewed five horizontally aligned arrowheads, half of the arrows being compatible (“>>>>>” or “<<<<<”) and the other half being incompatible (“>><>>” or “<<><<”). Participants were then told to quickly and accurately indicate the direction of the center arrow by clicking the right or left mouse buttons. Stimuli were presented for 200 ms, followed by a white fixation cross-centrally presented on a black background. Participants received up to 1,800 ms after the offset of the arrows to respond followed by an inter-trial interval that varied randomly between 1,000 and 2,000 ms. The task consisted of 11 blocks of 30 trials (330 total trials) interspersed with self-timed breaks. Participants received feedback on their performance at the end of each block. If accuracy was ≤75%, the message “Please try to be more accurate” was presented; if accuracy was >90%, the message, “Please try to respond faster” was displayed; in all other cases, participants saw the message, “You’re doing a great job.”
2.3. Data Recording and Analysis
Continuous electroencephalogram (EEG) for each participant was recorded during the task using the ActiveTwo BioSemi system. 34 standard electrode sites were used (32 channels plus FCz and Iz), with two additional electrodes placed on each mastoid. The EEG was pre-amplified to improve the signal-to-noise ratio at each electrode. The data were digitized at 24-bit resolution with a Least Significant Bit (LSB) value of 31.25 nV and a sampling rate of 1024 Hz, using a low-pass fifth order sinc filter with a −3 dB cutoff point at 204.8 Hz.
Artifact analysis was visually conducted via BVA 2 software (Brain Vison Software), with any channels that contained artifacts rejected on a trial-to-trial basis. Data were re-referenced to the average of the two mastoids and high-pass (0.1Hz) and low-pass (30Hz) filters. Standard eyeblink and ocular corrections were performed utilizing the Gratton & Coles algorithm which corrects ocular artifacts by using a regression based approach (Miller, Gratton, & Yee, 1988). Data were segmented beginning 500 ms before each response onset and continuing for 1500 ms. Baseline correction for each trial was performed using the 500 to 300ms prior to response onset. The ERN and CRN (correct response negativity) were scored as the average activity on error and correct trials, respectively, from 0 to 100 ms after response at a pooling of electrodes on the Fz, Cz, and Fcz electrode sites.
We calculated standardized residual scores for ERN and CRN, consistent with the recommendations of Meyer et al. (2017) suggesting that residual scores increase specificity by isolating variability in these measures. More specifically, the residual scores of ERN and CRN are less correlated compared to the raw ERN and CRN variables and the ΔERN (ERN-CRN), which is highly correlated in opposite directions with ERN and CRN. The ERNresid was calculated by saving the variance leftover in a regression where the CRN was entered predicting the ERN. Similarly, the CRNresid was calculated by saving the variance leftover in a regression where the ERN was entered predicting the CRN. The ERNresid and CRNresid were treated as two separate dependent variables in order to examine relations regarding neural response on error and correct trials.
Accuracy data were calculated as the percentage of trials that were correct. Reaction time was calculated as the amount of time it took participants to respond from the onset of the stimulus, separately for error and correct trials.
Separate stepwise linear regressions were conducted in SPSS with the ERNresid or CRNresid serving as dependent variables. The regression model included combat experiences (DRRI-2-Section D or CES). We also included anxiety symptoms (BAI) as a covariate given the robust relation between anxiety symptoms and the ERN (Weinberg et al., 2015). Identical regression models were conducted utilizing the DRRI-2-Sections A and N to assess pre- and post- deployment stressors.
3. Results
Table 1 provides descriptive and demographic information regarding the sample. Of the 32 veterans diagnosed with current PTSD, 71.8% had comorbid anxiety or depression. Bivariate correlations of the study variables are presented in Table 2. In regard to behavioral performance, participants committed an average of 29.7 (SD = 22.2, Range = 6–93) errors and correctly responded on 88.8% (SD = 8.9%) of trials. As expected, reaction times were faster for errors than for correct responses, t(67)=8.90, p<0.001.
Table 2.
Bivariate Correlations of Study Variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| Age | - | ||||||||
| CAPS | ‒.07 | - | |||||||
| BAI | ‒.10 | .69*** | - | ||||||
| DRRI-2 CE | ‒.21 | .46*** | .35** | - | |||||
| CES | ‒.06 | .52*** | .36** | .78*** | - | ||||
| ERNresid (uv) | .12 | .25 | ‒.15 | ‒.26* | ‒.11 | - | |||
| CRNresid (uV) | ‒.05 | ‒.19 | .25 | ‒.03 | ‒.13 | ‒.31* | - | ||
| Error RT | .27* | .21 | .01 | ‒.05 | .14 | .33* | ‒.38* | - | |
| Correct RT | .19 | .26* | .01 | .06 | .24 | .07 | ‒.30** | .53*** | - |
| Accuracy | .03 | ‒.33* | ‒.13 | ‒.21 | ‒.24 | .06 | .29* | ‒.24* | ‒.58*** |
Note: CAPS = Clinician-Administered PTSD Scale; BAI = Beck Anxiety Inventory; DRRI-2 = Deployment Risk and Resiliency Inventory – Combat Experiences Scale; CES = Combat Exposure Scale; ERNresid = Error Related Negativity Residual Score; CRNresid = Correct Response Negativity Residual Score; RT = Response Time;
= p < .001;
= p < .005;
= p < .05
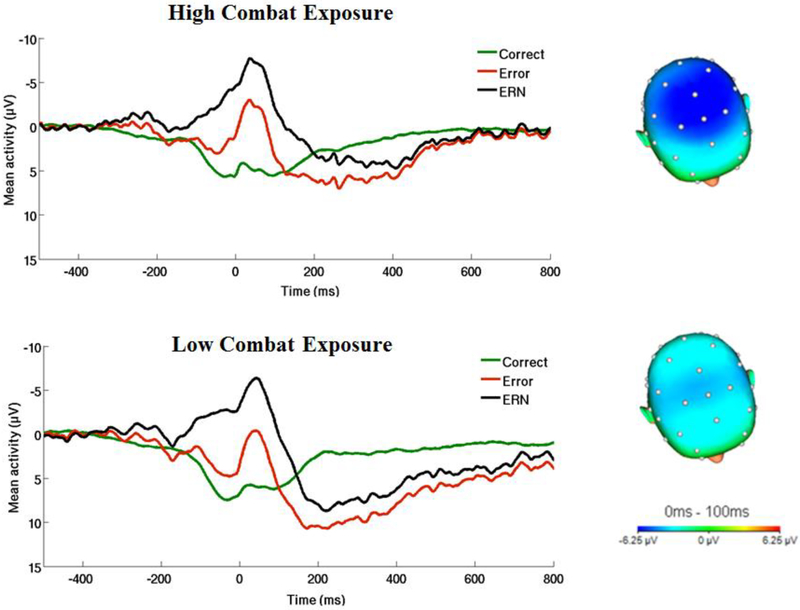
Next, we examined the influence of PTSD symptoms and combat experiences (DRRI-2 and CES in separate models) on the ERNresid. When utilizing the DRRI-2 to assess combat experiences, results revealed a main effect of combat experiences on the ERNresid, t(2,65) = −2.65, p = .01, β = −.34, reffect size= .31.1 As shown in Figure 2, veterans experiencing greater combat-related events exhibited a more enhanced ERN. When utilizing the CES to assess combat experiences, results revealed no relation between the ERNresid and combat exposure, t(2,65) = −1.21, p = .26, β = −.20, reffect size= .14.
Figure 2.
On the left, response-locked ERP waveforms for correct and error trials, as well as the difference waves (error-related negativity; ΔERN) and on the right, topographic maps of activity (error minus correct) for individuals with a) high and b) low combat-related exposure (defined by a median split). Combat experiences assessed from Section D of the Deployment Risk and Resilience Inventory-2.
To determine whether the influence of combat experiences was specific to the ERNresid, we conducted identical regression analyses with the CRNresid serving as the dependent variable. Results revealed no main effects when utilizing the CES, t(2,65) = −0.61, p = .55, β = −.08, reffect size= .07, or DRRI-2, t(2,65) = 0.28, p = 0.78, β = 0.04, reffect size= .03, to capture combat experiences. Finally, we examined if the effect of ERN amplitude was unique to combat exposure or if it was also significantly related to pre- and post-deployment stressors. These analyses yielded no significant main effects (lowest p = .17).
4. Discussion
The current study aimed to assess the association between combat exposure and ERN amplitude in a sample of military veterans returning from OEF/OIF/OND. We also examined whether the association between combat exposure and ERN response was influenced by the severity of PTSD symptoms in veterans. As predicted, veterans experiencing a greater number of combat-related events, as measured via the DRRI-2, were characterized by greater ERN amplitude. Importantly, this finding was maintained when adjusting for broad anxiety symptoms, suggesting that the relation between ERN and combat exposure is not better accounted for by internalizing psychopathology. Finally, results revealed no significant association between ERN amplitude and pre- and post-deployment life stressors, suggesting that the current set of findings appear to be specific for combat trauma and not observed for other forms of life stress among a sample of military veterans returning from OEF/OIF/OND.
Notably, previous studies have failed to find a significant difference in ERN amplitude between healthy controls and combat exposed veterans with PTSD (Rabinak et al., 2013; Swick, Honzel, & Turken, 2015). The current study highlights the importance of assessing degree of combat exposure when considering its relation to neural sensitivity to threat. The association between combat exposure, assessed by the DRRI-2, and ERN may suggest that veterans exposed to combat events become particularly reactive to the commission of errors. As previously highlighted, given the inherently dangerous nature of a war-zone, errors committed while in a combat zone could be deadly. As a result, veterans may develop a learned sensitivity to the commission of errors, which increases the ERN amplitude and facilitates survival during deployment. This interpretation aligns with previous work showing that punishment enhances ERN amplitude (Meyer & Gawlowska, 2017; Riesel, Weinberg, Endrass, Kathmann, & Hajcak, 2012). At the same time, in non-combat settings such as ones’ home environment, an enhanced ERN could be problematic. Upon returning home from war, veterans with an enhanced ERN amplitude may demonstrate overactive performance monitoring. They may also perceive errors to be especially catastrophic and experience high levels of distress in response to making mistakes, which further potentiates anxiety and sustains hypervigilance (Weinberg et al., 2015, 2016). In addition to potentially diminishing quality of life, increased anxiety could further promote maladaptive coping behaviors including drug and alcohol use, social isolation, and avoidance. Taken together, although greater ERN may be an adaptive response during times of combat, the persistence of an enhanced ERN upon returning home from war may be detrimental for certain veterans and associated with a host of poor outcomes.
We found limited evidence for an association between combat exposure and ERN amplitude when utilizing the CES to assess combat experiences. Although the CES and DRRI-2 were strongly correlated in the current study, the shared variance among these two measures was only approximately 54%. Relative to the CES, the DRRI-2 was developed later to reflect the experiences of deployment to OEF/OIF/OND (Vogt et al., 2013) and includes a greater number of specific items reflecting combat experiences in Afghanistan and Iraq (i.e., “I was involved in searching or clearing homes, buildings, or other locations”, “I was involved in locating or disarming explosive devices”, “I encountered land or water mines, booby traps, or roadside bombs (for example, IEDs)”. Although the CES has been more widely used in the literature, the current study highlights the utility of the DRRI-2 to assess combat experiences in OEF/OIF/OND veterans and suggests that the association between ERN amplitude and combat exposure may be influenced by how closely the items of the measure characterize the stressful experience.
There are several limitations of the current study that should be taken into consideration. First, all the participants in our study were male veterans, hindering us from generalizing our findings to women exposed to combat environments. Second, the study did not collect ERN data from veterans prior to their combat exposure during OEF/OIF/OND; therefore, future prospective studies are needed to directly test whether combat exposure predicts changes in the amplitude of the ERN. Third, given that the current study was cross-sectional, our findings are correlational and causation cannot be inferred. Next, although the relation between combat exposure and ERN remained significant after adjusting for symptoms of anxiety utilizing the BAI, future studies are needed to determine if this finding is maintained when controlling for more specific subtypes of anxiety (i.e., fear-based anxiety, worry) and diagnoses (i.e., generalized anxiety, social anxiety) that have been linked to the ERN (Gorka et al., 2017; Weinberg et al., 2015). Similarly, in order to increase current knowledge regarding ERN development, it will be important for future research to explore the extent to which other forms of trauma or negative experience (e.g., child abuse) influence the ERN. Last, future longitudinal studies are needed to directly test whether the ERN mediates the relation between level of combat exposure and the subsequent development of internalizing disorders.
In summary, our study demonstrates that greater combat exposure is associated with an enhanced ERN among OEF/OIF/OND veterans. Stressful experiences during combat may facilitate this neural propensity to overreact to the commission of errors, and this may persist post-deployment after soldiers have returned from war. Enhanced threat responding may therefore be one mechanism of heightened risk for psychopathology among combat-exposed veterans. Although future longitudinal projects are needed to directly test this possibility, the ERN may prove to be an important biological screening tool for the identification of veterans at risk for the development of psychopathology.
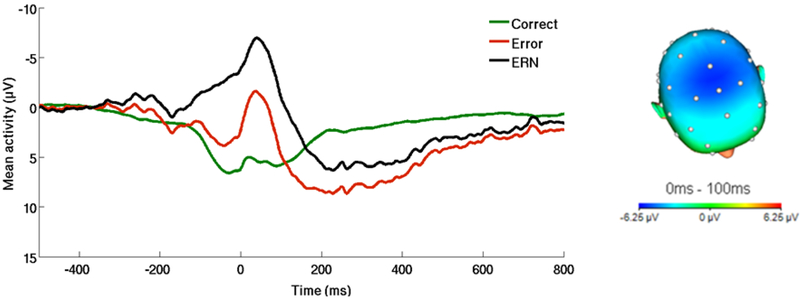
Figure 1.
On the left, response-locked ERP waveform for correct and error trials, as well as the difference waves (error-related negativity; ΔERN) across the entire sample. On the right, topographic map of activity (error minus correct) across the entire sample.
Funding
This study was funded by Veterans Affairs Merit Review Program Awards (I01CX000913 to KLP) from Clinical Sciences Research and Development, Office of Research and Development of the U.S. Department of Veterans Affairs. KLB is supported by National Institute of Mental Health Grant K23-MH113793. SMG is supported by National Institute of Alcohol Abuse and Alcoholism Grant K23-AA025111.
Footnotes
This relation was also observed for the non-residualized ERN variable and ΔERN (error minus correct difference score).
References
- Bar-Haim Y, Holoshitz Y, Eldar S, Frenkel TI, Muller D, Charney DS, … & Wald I (2010). Life-threatening danger and suppression of attention bias to threat. American Journal of Psychiatry, 167(6), 694–698. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, & Keane TM (1995). The development of a clinician-administered PTSD scale. Journal of Traumatic Stress, 8(1), 75–90. [DOI] [PubMed] [Google Scholar]
- Endrass T, Schuermann B, Kaufmann C, Spielberg R, Kniesche R, & Kathmann N (2010). Performance monitoring and error significance in patients with obsessive-compulsive disorder. Biological Psychology, 84(2), 257–263. [DOI] [PubMed] [Google Scholar]
- Gorka SM, Burkhouse KL, Afshar K, & Phan KL (2017). Error-related brain activity and internalizing disorder symptom dimensions in depression and anxiety. Depression and anxiety, 34(11), 985–995. [DOI] [PubMed] [Google Scholar]
- Gorka SM, MacNamara A, Aase DM, Proescher E, Greenstein JE, Walters R, … & DiGangi JA (2016). Impact of alcohol use disorder comorbidity on defensive reactivity to errors in veterans with posttraumatic stress disorder. Psychology of Addictive Behaviors, 30(7), 733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, & Foti D (2008). Errors are aversive: Defensive motivation and the error-related negativity. Psychological Science, 19, 103–108. [DOI] [PubMed] [Google Scholar]
- Hoge CW, Auchterlonie JL & Milliken CS (2006). Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA Psychiatry, 295, 1023–1032. [DOI] [PubMed] [Google Scholar]
- Keane TM, Fairbank JA, Caddell JM, Zimering RT, Taylor KL, & Mora CA (1989). Clinical evaluation of a measure to assess combat exposure. Psychological Assessment, 1(1), 53–55. [Google Scholar]
- Meyer A, & Gawlowska M (2017). Evidence for specificity of the impact of punishment on error-related brain activity in high versus low trait anxious individuals. International Journal of Psychophysiology, 120, 157–163. [DOI] [PubMed] [Google Scholar]
- Meyer A, Hajcak G, Glenn CR, Kujawa AJ, & Klein DN (2017). Error-related brain activity is related to aversive potentiation of the startle response in children, but only the ERN is associated with anxiety disorders. Emotion, 17(3), 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Lerner MD, De Los Reyes A, Laird RD, & Hajcak G (2017). Considering ERP difference scores as individual difference measures: Issues with subtraction and alternative approaches. Psychophysiology, 54(1), 114–122. [DOI] [PubMed] [Google Scholar]
- Miller GA, Gratton G, & Yee CM (1988). Generalized implementation of an eye movement correction procedure. Psychophysiology, 25(2), 241–243. [Google Scholar]
- Moser JS, Hajcak G, & Simons RF (2005). The effects of fear on performance monitoring and attentional allocation. Psychophysiology, 42, 261–268. [DOI] [PubMed] [Google Scholar]
- Olvet DM, & Hajcak G (2011). The error-related negativity relates to sadness following mood induction among individuals with high neuroticism. Social Cognitive and Affective Neuroscience, 7(3), 289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Holman A, Angstadt M, Kennedy AE, Hajcak G, & Phan KL (2013). Neural response to errors in combat-exposed returning veterans with and without post-traumatic stress disorder: A preliminary event-related potential study. Psychiatry Research: Neuroimaging, 213(1), 71–78. [DOI] [PubMed] [Google Scholar]
- Riesel A, Weinberg A, Endrass T, Kathmann N, & Hajcak G (2012). Punishment has a lasting impact on error-related brain activity. Psychophysiology, 49(2), 239–247. [DOI] [PubMed] [Google Scholar]
- Sheehan D, Lecrubier Y, Sheehan KH, Sheehan K, Amorim P, Janavs J, … & Dunbar G (1998). Diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry, 59, 22–33. [PubMed] [Google Scholar]
- Swick D, Honzel N, & Turken U (2015). Intact error monitoring in combat Veterans with post-traumatic stress disorder. Psychiatry Research: Neuroimaging, 234(2), 227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, & Marusak HA (2017). Toward understanding the impact of trauma on the early developing human brain. Neuroscience, 342, 55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt D, Smith BN, King LA, King DW, Knight J, & Vasterling JJ (2013). Deployment risk and resilience inventory-2 (DRRI-2): An updated tool for assessing psychosocial risk and resilience factors among service members and veterans. Journal of Traumatic Stress, 26(6), 710–717. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Dieterich R, & Riesel A (2015). Error-related brain activity in the age of RDoC: a review of the literature. International Journal of Psychophysiology, 98(2), 276–299. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Meyer A, Hale-Rude E, Perlman G, Kotov R, Klein DN, & Hajcak G (2016). Error-related negativity (ERN) and sustained threat: Conceptual framework and empirical evaluation in an adolescent sample. Psychophysiology, 53(3), 372–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Riesel A, & Hajcak G (2012). Integrating multiple perspectives on error-related brain activity: The ERN as a neural indicator of trait defensive reactivity. Motivation and Emotion, 36, 84–100. [Google Scholar]