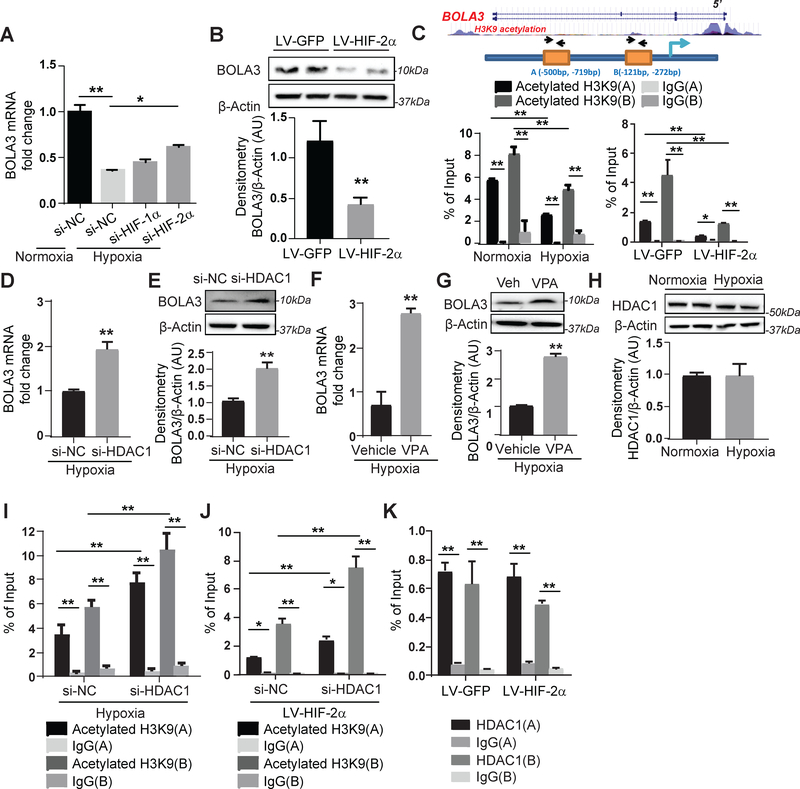
Figure 2: Hypoxia mediates transcriptional repression of BOLA3 via HIF-2α/HDAC/histone acetylation pathway.
(A) Transfection of siRNA targeting hypoxia induced factor (HIF)-2α, but not HIF-1α, partially rescued BOLA3 expression in hypoxic human PAECs. (B) Lentivirus (LV)-mediated forced expression of a constitutively active HIF-2α in PAECs inhibited BOLA3. (C) ChIP-qPCR via immunoprecipitation of acetylated histone 3 lysine 9 (H3K9) and PCR detection of BOLA3 promoter sites indicated acetylated H3K9 enrichment of the BOLA3 promoter (sites A, B), which was decreased by hypoxia (left) or constitutive HIF-2α (right). (D-G) siRNA knockdown of histone deacetylase 1 (HDAC1) as well as valproic acid (VPA, 3mM), a HDAC inhibitor, both rescued BOLA3 transcript and protein expression in hypoxic PAECs. (H) Expression of HDAC1 remained unchanged in hypoxic PAECs. (I-J) siRNA knockdown of HDAC1 enhanced enrichment of H3K9 acetylation at the BOLA3 promotor in hypoxia (I) or with constitutive HIF-2α expression (J). (K) ChIP-qPCR via immunoprecipitation of HDAC1 indicated HDAC1 enrichment at BOLA3 promoter sites that was not altered with constitutive HIF-2α expression. In all panels measuring fold change, mean expression in control groups (si-NC, LV-GFP, normoxia, vehicle) was normalized to fold change of 1, to which relevant samples were compared (N = 3/group). Data represent the mean ± SEM (*P < 0.05, **P < 0.01).