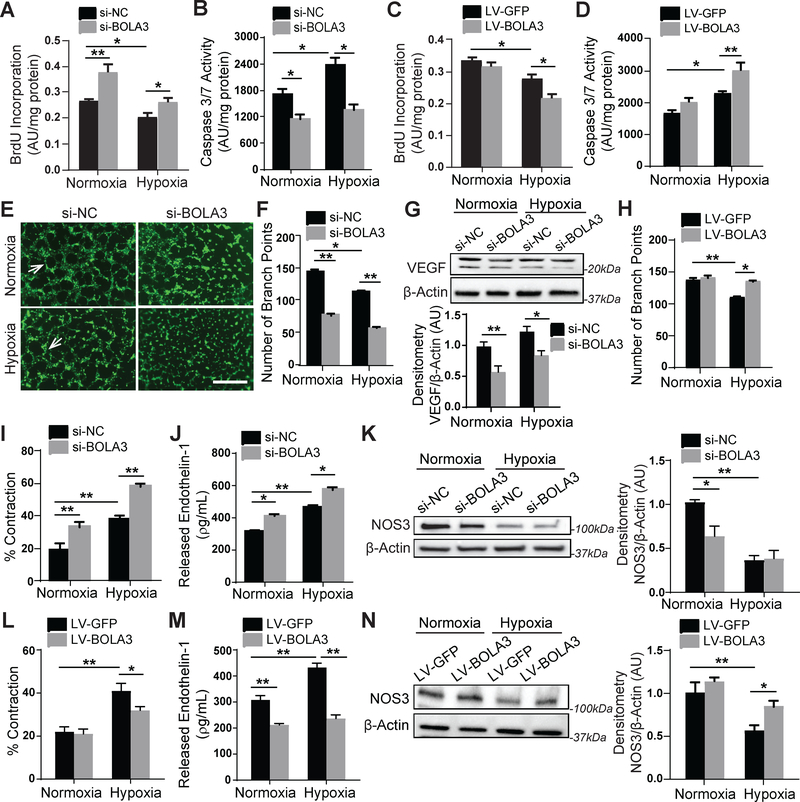
Figure 5: BOLA3 deficiency increases proliferation, inhibits apoptosis and angiogenesis, and promotes vasoconstriction in PAECs.
(A-B) As reflected by BrdU incorporation and apoptotic caspase 3/7 activation, BOLA3 knockdown increased PAEC proliferation and reduced apoptosis in both normoxia and hypoxia. (C-D) Conversely, forced BOLA3 expression inhibited proliferation and promoted apoptotic signaling in hypoxia. (E-F) BOLA3 knockdown inhibited in vitro angiogenic potential, as measured by tube formation in matrix gel in normoxia and hypoxia (E), white arrows indicate representative full tubes and branch point quantification (F). Scale bar, 200μm. (G) BOLA3 knockdown down-regulated vascular endothelial growth factor (VEGF) as assessed by immunoblot. (H) Conversely, forced BOLA3 expression increased in vitro tube formation in hypoxic PAECs. (I) As quantified by a gel matrix contraction assay encompassing the exposure of PASMCs in gel matrix to conditioned serum-free medium from PAECs, knockdown of BOLA3 in PAECs produced conditioned media that increased PASMC contraction in normoxia and hypoxia. (J) As quantified by ELISA, BOLA3 knockdown increased secreted endothelin-1 (ET-1) production in normoxia and hypoxia. (K) As assessed by immunoblot and densitometry, BOLA3 knockdown in normoxia inhibited nitric oxide synthase 3 (NOS3) expression, at least partially phenocopying the more robust downregulation in hypoxia. (L) Conditioned serum-free medium from PAECs constitutively expressing BOLA3 transgene blunted the hypoxic induction of PASMC contraction in matrix gel. (M) Forced BOLA3 expression inhibited ET-1 release in normoxia and hypoxia. (N) More evident in hypoxia, constitutive BOLA3 transgene expression rescued NOS3 expression. In panels G, K, and N, mean expression of control group (Nx si-NC or Nx LV-GFP) was normalized to fold change of 1, to which relevant samples were compared. For all panels, N = 3–6 replicates/group. Data represent the mean ± SEM (*P < 0.05, **P < 0.01).