Figure 2.
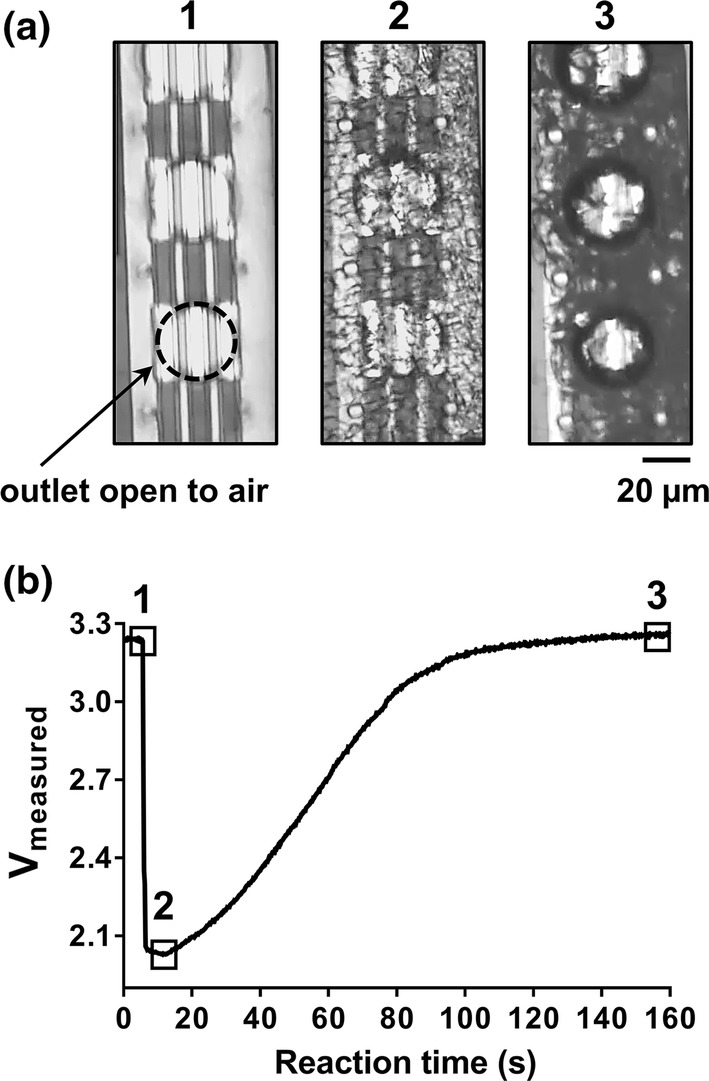
Microfluidic platform with anticoagulated whole human blood sample. Venous whole blood samples collected from a healthy human donor (Hematocrit of 43) were anticoagulated with bivalent direct thrombin inhibitor (40 µg/mL hirudin) and applied to the inlet of the microfluidic device. Real-time progression of blood sample within microfluidic device was (a) visualized with light microscopy with each pertinent transition point shown in panels (1–3) and (b) labeled on the voltage profile (1) dry sensing zone prior to blood entry, (2) entry of blood samples and (3) sedimentation of RBCs within sensing zone. Data representative of n > 30.
