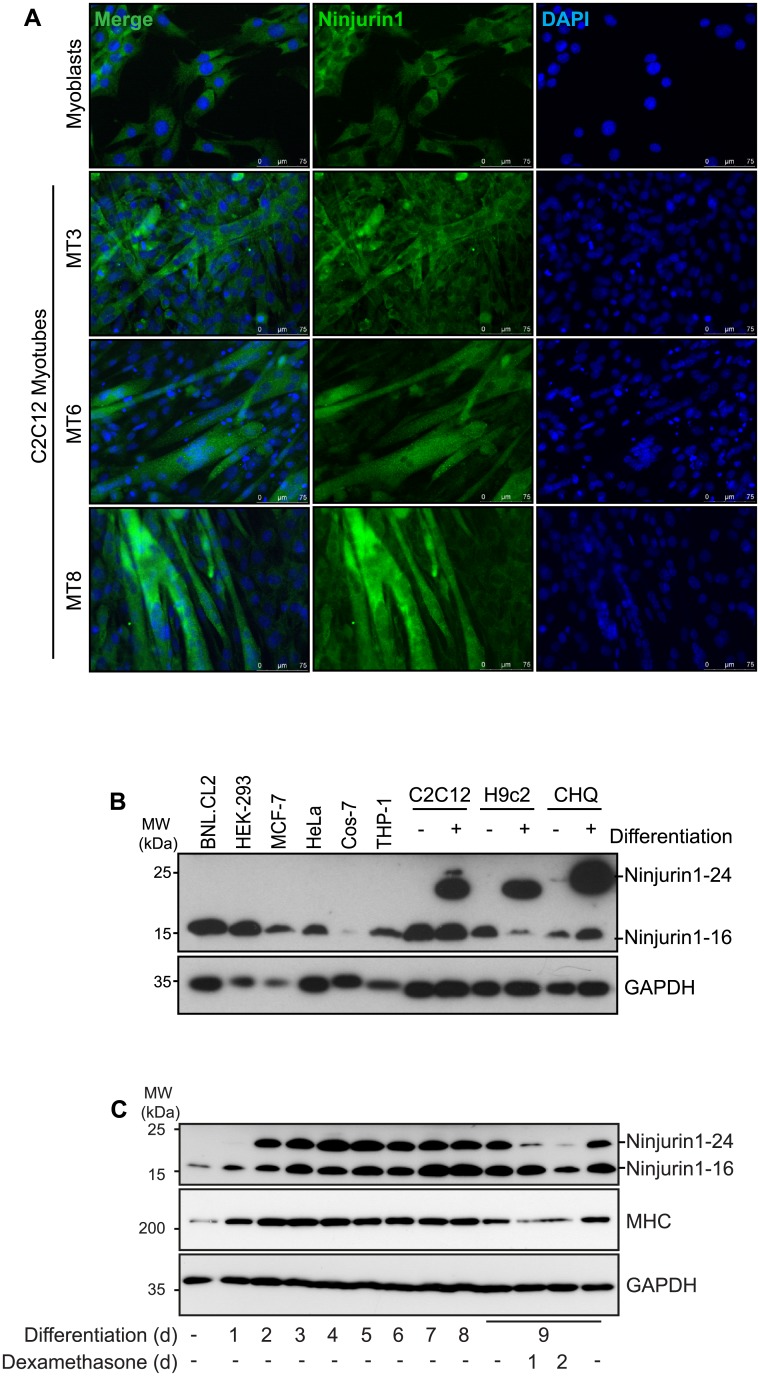
Fig 5. Ninjurin1 increases during myogenic differentiation in skeletal myocytes.
(A) Immunofluorescent staining of C2C12 myoblasts, and for 3, 6 and 8 days differentiated myotubes, as indicated (MT3, MT6, MT8), with anti-Ninjurin1 as primary antibody and Alexa Fluor 488 conjugated secondary antibody (green). Nuclei were stained with DAPI (blue). Scale bar, 75 μm. (B) Western blots of proteins isolated from different non-myocyte (BNLCL2, HEK-293, MCF-7, HeLa, Cos-7, THP-1) and myocyte (C2C12, H9c2, CHQ) cell lines (as indicated) using anti-Ninjurin1 antibody. The myocyte cell lines C2C12, H9c2 and CHQ were analyzed as undifferentiated myoblasts (-) and differentiated myotubes (+). GAPDH was used as loading control. The 16kDa (Ninjurin1-16) and 24kDa (Ninjurin1-24) Ninjurin1 variants are indicated. (C) Western blots of proteins isolated from C2C12 myoblasts and for different time points (as indicated) differentiated myotubes using anti-Ninjurin1 antibody. To induce atrophy 9 days differentiated C2C12 myotubes were treated with Dexamethasone (10 μM) for 1 and 2 days, as indicated. GAPDH was used as loading control. The 16kDa (Ninjurin1-16) and 24kDa (Ninjurin1-24) Ninjurin1 isoforms are indicated. MHC indicates myosin heavy chain.