Fig. 1. PES1 directly interacts with hTERT.
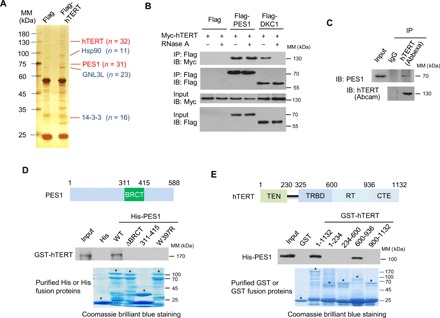
(A) Cell extracts from MCF7 cells stably expressing Flag (control) or Flag-tagged hTERT (Flag-hTERT) were immunopurified with anti-Flag affinity gel, and the purified complex was subject to SDS–polyacrylamide gel electrophoresis (PAGE) and silver-stained. The differential protein bands were retrieved and analyzed by MS. n, number of peptides identified by MS analysis; MM, molecular mass. (B) HEK293T cells transiently transfected with Myc-tagged hTERT (Myc-hTERT) and Flag-PES1 or Flag-DKC1 were treated with or without RNase A (0.1 mg/ml), and immunoprecipitated (IP) with anti-Flag, followed by immunoblotting (IB) with the indicated antibodies. (C) MCF7 cell lysates were immunoprecipitated with anti-hTERT from Abbexa and immunoblotted with anti-hTERT from Abcam and anti-PES1. IgG, immunoglobulin G. (D) Glutathione-Sepharose beads bound with GST-hTERT were incubated with purified His-tagged wild-type (WT) or mutated PES1. After washing the beads, the bound proteins were subjected to SDS-PAGE and immunoblotted with anti-GST antibody. Also shown on top of the graph is a schematic diagram of the PES1 protein, illustrating the location of the BRCT domain. Asterisks indicate the positions of the expected full-length fusion proteins. (E) Glutathione-Sepharose beads bound with WT or truncated hTERT were incubated with purified His-tagged PES1. After washing the beads, the bound proteins were examined by immunoblot with anti-His. Schematic diagram of the hTERT deletion constructs used is shown at the top. Asterisks indicate the positions of the expected full-length fusion proteins. TEN, telomerase N-terminal; TRBD, TR-binding domain; RT, reverse transcriptase; CTE, C-terminal extension.
