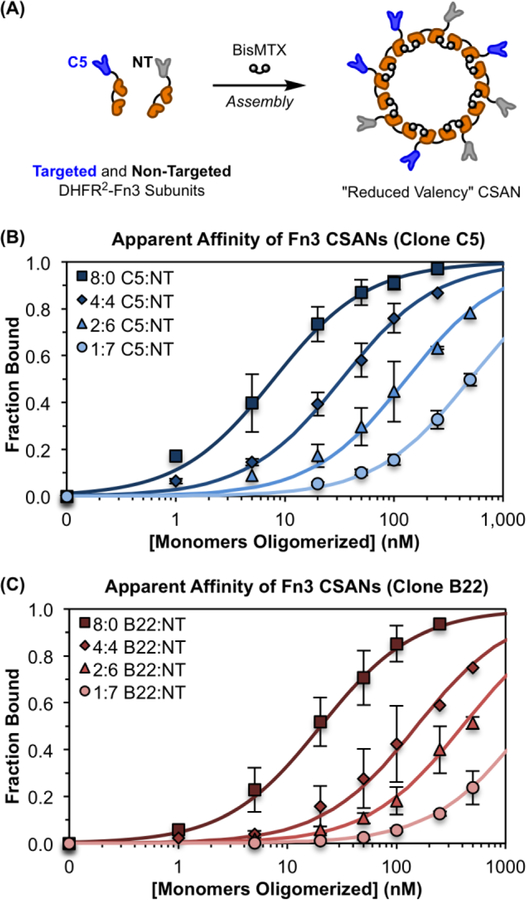
Figure 2. Modular Assembly of CSANs Enables Affinity Tuning.
(A) CSANs with a reduced valency of EpCAM-binding Fn3 domains can be formed by co-assembling defined molar ratios of targeted and non-targeted DHFR2-Fn3 fusion proteins using the chemical dimerizer, bisMTX. This was done for both the “high-affinity” Fn3 clone C5 and the “low-affinity” Fn3 clone B22. (B&C) CSANs of distinct average valencies were titrated against MCF-7 cells via flow cytometry to determine their apparent affinity for cellular EpCAM, as previously described.43, 46 The apparent affinity of both scaffolds, (B) C5-targeted CSANs and (C) B22-targeted CSANs, varied directly with the proportion of binding domains in the CSAN. The legends in (B) and (C) give the ratio of targeted to non-targeted (T:NT) subunits incorporated into each CSAN. The x-axis provides the total concentration of DHFR2-Fn3 monomers that were oligomerized into CSANs using bisMTX. Each sample is normalized to the fitted maximum fluorescence intensity for that sample, enabling determination of the fraction of bound ligand at each concentration. Un-normalized binding isotherms are provided in Figure S4. Each titration was performed at least three independent times, and data is presented as the mean ± standard deviation of these trials.