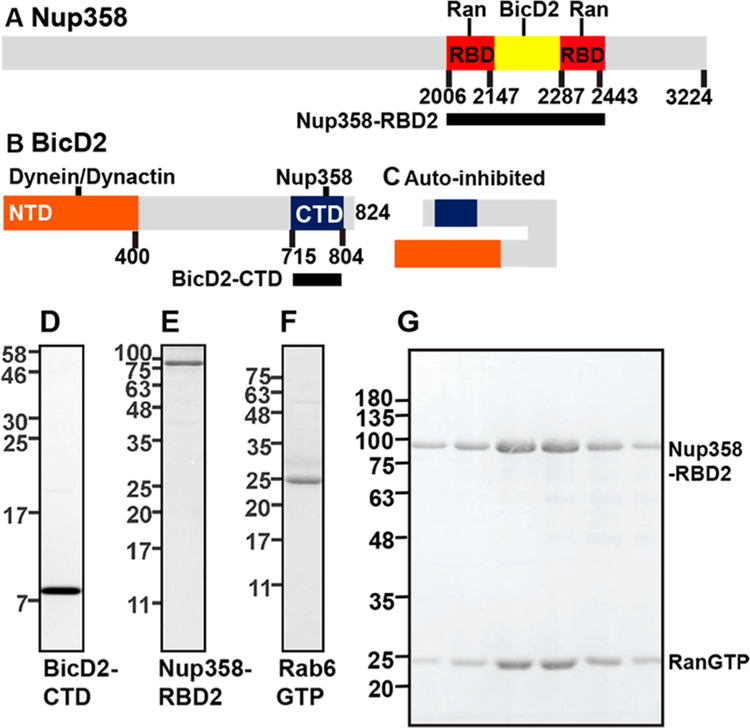
Figure 1.
Purification of the cargo binding domain of BicD2 and its cargoes. (A) For biophysical studies, Nup358-RBD2 (black bar), a fragment of Nup358 that includes the BicD2 binding site and the two adjacent Ran binding domains (RBD red), was purified. (B) The N-terminal domain (NTD, orange) of BicD2 interacts with dynein/dynactin, while the CTD (blue) recruits cargo.2,11 For biophysical studies, the BicD2-CTD was purified (black bar). (C) In absence of cargo, the NTD and CTD of BicD2 form an autoinhibited state that cannot recruit dynein/dynactin.2,5,9,10 (D−F) SDS-PAGE analysis of purified proteins is shown. Masses of molecular weight standards in kDa are indicated on the left. (D) BicD2-CTD. (E) Nup358-RBD2. (F) Rab6GTP. (G) Purification of a RanGTP/Nup358 complex. Purified Nup358-RBD2 and RanGTP were mixed. Nup358-RBD2 with RanGTP bound was separated by gel filtration. SDS-PAGE analysis of the elution fractions is shown.