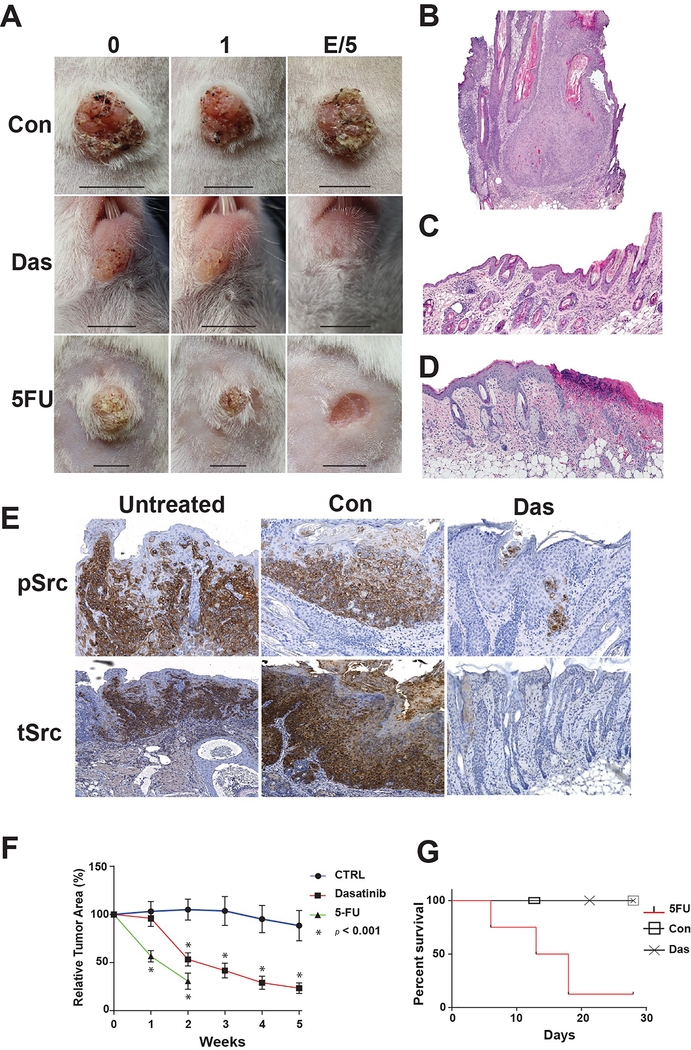
Figure 1. Topical Dasatinib induces cSCC regression and inhibits Src oncodrivers without ulceration or mortality.
A) Representative images of cSCCs in K14-Fyn Y528F mice treated with vehicle (Con), Dasatinib (Das) or 5-FU taken at time 0, 1 week or 5 weeks/treatment endpoint. Scale bar- 5 mm B-D) Representative histologic images of cSCCs at the treatment endpoints: Vehicle (B), Dasatinib (C) or 5-FU (D). E) Immunostaining for activated Src kinases and total Src kinases (tSrc) was performed on representative sections of untreated, vehicle-treated (Con) or dasatinib-treated (Das) cSCCs. Near complete loss of activated Src kinases was noted in dasatinib-treated, regressing cSCCs. Immunostaining for total Src kinases demonstrated a similar, but broader staining pattern. F) Relative tumor area as a function of treatment and time. Vehicle-treated cSCCs, N=18, grow and persist while dasatinib-treated cSCCs demonstrate regression by week 2, N=25. 5-FU treated lesions demonstrate significant regression by week 1, N=13. Error bars indicate SEM. * p < 0.05 G) Mice treated with 5-FU demonstrate high mortality by 18 days. No mortality noted in vehicle or dasatinib treated cohorts.