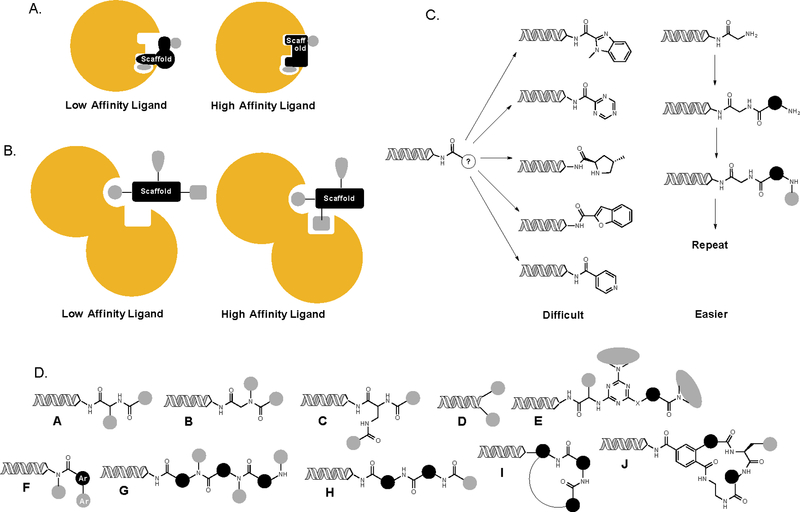
Fig. 2. Scaffold diversity.
A. Scaffolds (black) in low molecular weight (MW) molecules usually make critical contacts with the target biomolecule in addition to positioning appendages in a given three-dimensional orientation. Thus, it is important to have chemically diverse core scaffolds in libraries of low MW molecules. B. In higher MW foldamer-type molecules, the scaffold is not expected to engage the target biomolecule directly. Here, the critical issue is to hold appendages in relatively inflexible and distinct three-dimensional orientations. C. Scaffold diversity, especially with respect to heterocyclic cores, is difficult to achieve in split and pool chemistry due to chemical limitations. It is much easier to construct stiff building blocks onto which appendages can be grafted and oligomerize these into diverse scaffolds. D. A graphical representation of several existing DELs. Many of the lower MW libraries (A-D) have essentially no scaffold diversity, whereas some contain several (E,F). Some scaffold-diverse oligomer libraries (G, H) have been reported, including macrocycles (I,J). Ar = aryl.