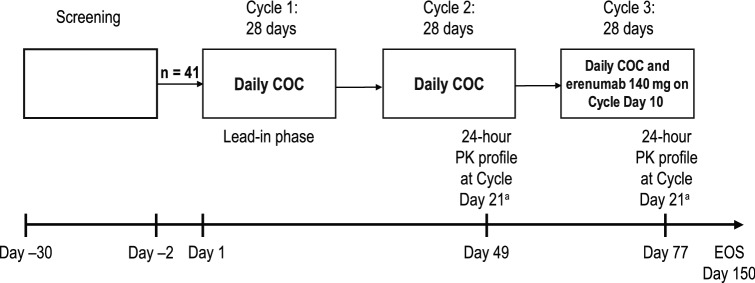
Fig. 1.
Study design and treatment schema. PK samples were collected on day 21, which was the day the last active dose of COC was administered and 11 days after erenumab administration (approximately the time of maximum erenumab concentration). COC was expected to be at steady state, offering the maximum potential to detect drug–drug interactions. COC combination oral contraceptive, EOS end of study, PK pharmacokinetics. aPK samples were collected on day 21, which was the day the last active dose of COC was administered and 11 days after erenumab administration (approximately the time of maximum erenumab concentration). COC was expected to be at steady state, offering the maximum potential to detect drug-drug interactions