Highlights
-
•
Single amygdala GABA neuron projects to both brain stem and cortex.
-
•
Bifurcating amygdala neurons may regulate emotions and muscle tone.
Keywords: AAV retro, GFP, tdTomato, CeA, BLA, VGAT-Cre mice
Abstract
The amygdala regulates multiple behaviors and emotions by projecting to multiple brain regions. However, the topographical distribution of amygdala neurons projecting to specific brain areas is still unclear. In the present study, we focus on determining whether single amygdala neurons project to the brain stem ventrolateral periaqueductal grey (vlPAG) and to the medial prefrontal cortex (mPFC). The mPFC neurons are involved in detecting emotional content while the vlPAG neurons are involved in regulating muscle tone. In VGAT-Cre mice a cre-inducible retrograde AAV tracer expressing tdTomato was microinjected into the ventrolateral periaqueductal grey matter (vlPAG), while a second retrograde AAV tracer with generic expression of GFP was delivered into the medial prefrontal cortex (mPFC). The results identified a subgroup of bifurcating GABAergic neurons in the central nucleus (CeA) and basolateral amygdala (BLA) that projects to vlPAG and mPFC. Based on these projections we suggest that amygdala GABA neurons may be involved in triggering emotionally-induced cataplexy in the sleep disorder, narcolepsy.
1. Introduction
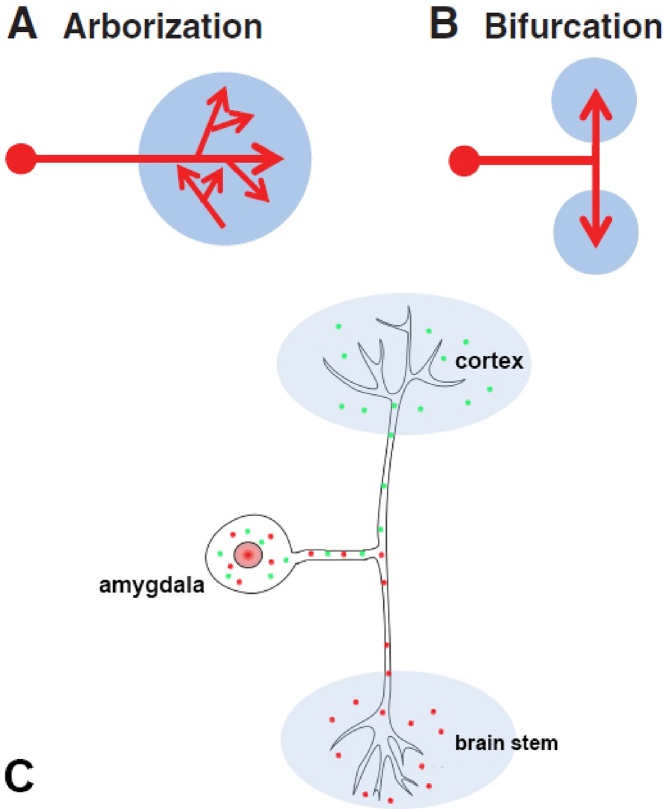
In this study we define “bifurcating” as a type of axonal branching in which branches arising from a single axon shaft are used for connecting the neuron to two distant targets located in opposite directions of the neuroaxis. This type of architectural neuronal connection is different from “arborization” whereby branching develops until the axon has reached a target distributing the neuronal synaptic contacts among nearby neurons (Fig. 1). Neurons may project to single target areas making axonal branching and arborization near their axon terminal. However, neurons may also send bifurcating projections to two distal targets (Shiromani, Floyd et al. 1990, Doron and Ledoux 2000, Katoh and Benedek 2003, Gibson and Ma 2011, Cao, Naito et al. 2012, Rockland 2013, Rockland 2018).
Fig. 1.
Common Axon branching processes in vertebrate nervous system. A: arborization occurs at the axon terminals in target site. B: bifurcation occurs at the axon main shaft with braches projecting to targets in opposite directions. C: a schematic drawing of an amygdala bifurcating neurons projecting to both cortex and brain stem. Green dots: pAAV-CAG-GFP; Red dots: pAAV-FLEX-tdTomato. (A and B are adjusted from Gibson et al., 2011).
To date bifurcating neurons, though were mentioned in a few studies, have not been systematically examined within the amygdala (Bienvenu, Busti et al. 2015, Zhang, Liu et al. 2019). Nonetheless bifurcating connections would be an efficient connection type for simultaneous integration of two distal regions in the CNS. Specifically, amygdala neurons featuring bifurcating axons could function as centers for synchronic modulation of two aspects of the adaptive emotional response. Adaptive emotional response is a very complex behavior demanding appropriate and fast integration of memory traces, ongoing perception (sensory), risk assessment (cognition) and appropriate action (motor output). For instance, risk assessment of a perceived threat could lead to a very different but very fast adaptive emotional response depending on the context. If a threat is sudden and in close proximity an adaptive response would be freezing behavior. In contrast, if a threat is close but there is opportunity to flee then escape would be a more adaptive behavior.
In this study we focused on determining whether single amygdala neurons project to two distal regions of the brain involved in regulation of two distinct aspects of the adaptive emotional response. One aspect is decision making and the other is motor output. The medial prefrontal cortex (mPFC) is chiefly involved in adaptive decision making (Rushworth and Behrens 2008) whereas the ventrolateral periaqueductal gray (vlPAG) in the midbrain is key to modulate the motor output of adaptive defensive responses (Tovote, Esposito et al. 2016, Motta, Carobrez et al. 2017). Amygdala neurons have been shown to project to either the vlPAG or mPFC (Marek, Strobel et al. 2013, Li and Sheets 2018). However, bifurcating neurons innervating both targets simultaneously have not been determined. Single amygdala neurons innervating both targets could integrate multiple functions harmoniously and quickly. Because one of the amygdala major outputs is GABAergic from CeA, we also felt compelled to describe in detail bifurcating projections from GABAergic amygdala neurons. To identify GABA neurons, we used vesicular GABA transporter (VGAT)-Cre transgenic line. Applying novel genetically controlled retrograde tracers that express two reporter genes into VGAT-Cre mouse identified a subgroup of bifurcating GABAergic amygdala neurons.
2. Materials and Methods
2.1. Animals and surgery
All manipulations done to the mice abide by the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Medical University of South Carolina IACUC (protocol #3267). VGAT-ires-Cre Knock-In mice (VGAT-Cre, www.jax.org, stock #016962) were used to target GABA neurons. All mice used were between 6-10 months old and both sexes were included. 6 mice (3 males and 3 females) were used for tracing. 2 wild type C57BL/6 mice were used as control to verify the specificity of the Cre-dependent vector. Retrograde tracers pAAV-CAG-GFP and pAAV-FLEX-tdTomato (Cre-dependent) were gifts from Edward Boyden (Addgene viral prep #37825-AAVrg and #28306-AAVrg, http://www.addgene.org/37825/, http://www.addgene.org/28306/).
Under deep anesthesia (isoflurane 2.0%) and using a stereotaxic instrument (Kopf, Tujunga, CA) retrograde AAV vectors with Cre inducible expression of tdTomato (pAAV-FLEX-tdTomato, Titer: 7 × 1012 genomic copy/ml) were microinjected into the left vlPAG area (A:-4.90 mm posterior to Bregma, L:0.80 mm, V:3.35 mm ventral to the skull (Hof et al., 2000). Vectors were delivered in a volume of 500 nl on left side of the brain using a 2.0 μL Hamilton syringe coupled to a 33-gauge stainless steel injector (Plastics One, Roanoke, VA). Injections were done gradually over 15 min. After the microinjection, the injector needle was left in place for 15 min and then withdrawn slowly. Similarly, retrograde AAV vectors with generic expression of GFP (pAAV-CAG-GFP, Titer: 7 × 1012 genomic copy/ml) were microinjected into the left mPFC (A:1.70 mm anterior to Bregma, L:0.40 mm, and V:1.2 mm ventral to brain surface (Hof et al., 2000). After surgery the animals were housed in Plexiglas cages with food and water available ad libitum. The ambient temperature was 23-25 °C and a 12 h light/dark cycle (6:00 A.M. to 6:00 P.M. lights on) was always maintained inside the housing room.
2.2. Histology and multiphoton laser scanning microscopy
21 days after injection mice were deeply anesthetized with isoflurane (5%) and were perfused transcardially with 0.9% saline (5–10 ml) followed by 10% buffered formalin in 0.1 M PBS (50 ml). The brains were sectioned at 40 μm thickness on a compresstome (Precisionary Instruments, Greenville, NC, USA). Coronal sections were scanned using a Leica multiphoton microscope (Leica TCS SP8-MP) to visualize tdTomato and GFP in the amygdala as well as in the injection sites. TdTomato and GFP positive cells will clear soma were counted on digitized images using MCID image analysis software (St. Catharines, ON, Canada).
3. Results
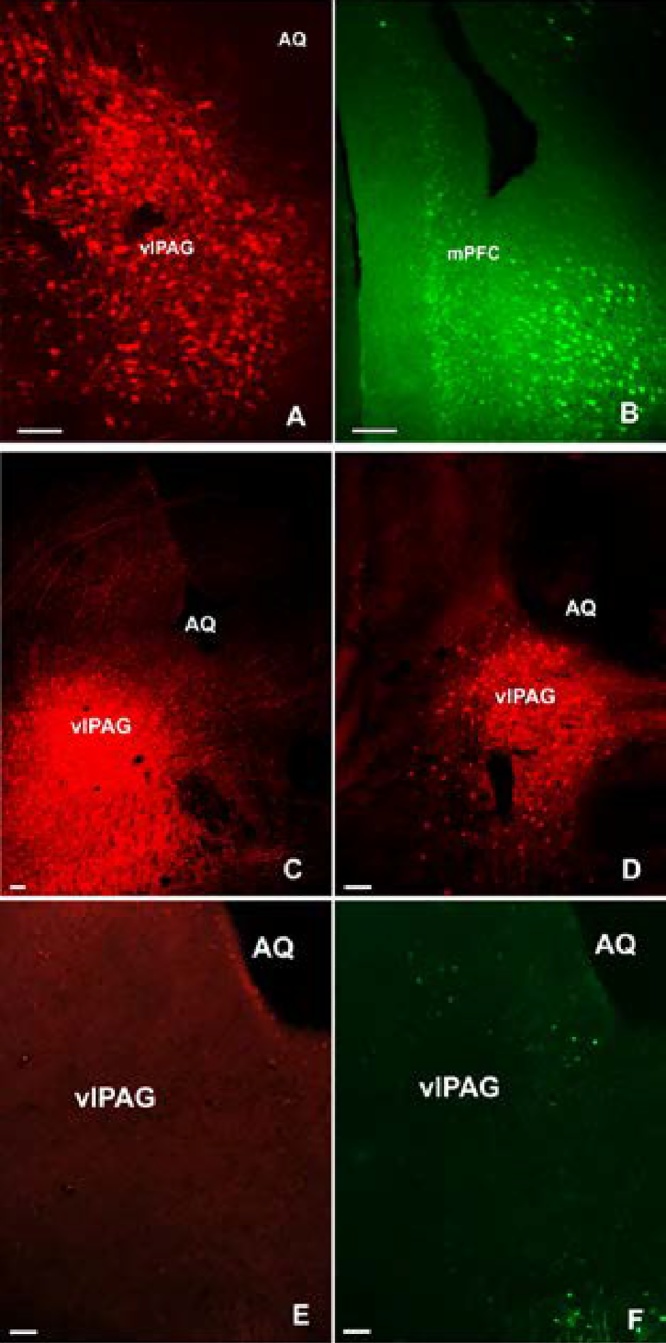
Fig. 2 shows the gene expression of tdTomato in the vlPAG area (A, C and D) and GFP in the mPFC area (B). In all three mice (2 males and 1 female) with correct vector targeting, the expression of both transgenes was exclusively seen within those two brain areas corroborating that the vectors were delivered at their correct targets. The positive transfected neurons observed at the vlPAG are likely to be GABAergic neurons since the pAAV-FLEX-tdTomato is a Cre-dependent viral vector. Tdtomato expression was absent in wild type C57BL/6 control mice (Fig. 2E and F).
Fig. 2.
The expression of tdTomato (A, C and D) and GFP (B) from AAV-retro vectors at the injection sites. Panel C and D showed the injection site at rostral (superior colliculus) level and caudal (inferior colliculus) level, respectively. In the vlPAG of wild type C57BL/6 mice, tdtomato expression was absent (E) while a few GFP positive cells were spotted. scale bar = 100 μm. AQ: aqueduct. mPFC: medial prefrontal cortex. vlPAG: ventrolateral periaqueductal gray.
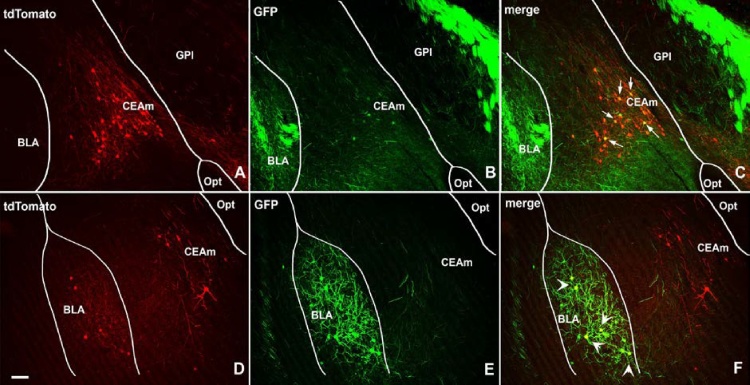
At the amygdala level, we found 283 and 174 retrogradely labeled neurons in the CeA and BLA, respectively. Outside of the CeA and the BLA there were only scattered retrograde labeled neurons (Fig. 3). Within the CeA the great majority (85% of retrograde labeled) neurons were found projecting to the vlPAG (tdTomato+). In the BLA a substantial portion (57%) of all labeled neurons projected to the vlPAG. Table 1 details that 70% of the retrograde labeled neurons in the CeA only connected with the vlPAG (tdTomato+/GFP-) whereas 15% only connected with the mPFC (GFP+/tTdomoto-). In the BLA, projection to the vlPAG but not to the mPFC (Tdtomato+/GFP-) made up a small percent (29%) of total labeled neurons. Projections to the mPFC but not to the vlPAG (GFP+/tdTomato-) made up 43%. Altogether 54% of all retrograde amygdala neurons (CeA + BLA) connected exclusively to the vlPAG (tdTomato+/GFP-). Since these neurons also expressed tdTomato we concluded these were GABAergic projections. On the other hand, roughly a quarter of all (26%) amygdala retrograde labeled neurons only connected to the mPFC (GFP+/tdTomato-).
Fig. 3.
Anatomical distributions of amygdala projecting neurons in the CeA (A-C) and in the BLA (D-E) revealed by genetic controlled retrograde tracing. Amygdala neurons projecting (tdTomato+; red labeled) to the vlPAG and neurons projecting (GFP+, green labeled) to the mPFC could be seen within both CeA and BLA. Interestingly double labeled neurons (yellow labeled) reveal the existence of many bifurcating neurons projecting to both vlPAG and mPFC. Bifurcating neurons can be seen within the CeA (indicated by arrows in C) as well as within the BLA (indicated by arrowheads in F). Scale bar = 100 μm. CeAm = medial aspect of the CeA, Opt = Optic chiasm, GPI = Globolus pallidus internus.
Table 1.
Tally of retrograde labelled amygdala neurons counted from four coronal sections (160 μm apart) from three VGAT mice
| Projecting to vlPAG; i.e. Td+/GFP- | Projecting to mPFC; i.e. GFP+/Td- | Projecting to both; i.e.Td+/GFP+ | |
|---|---|---|---|
| CeA | {53 79 66}198 [70%] | {12 13 18} 43 [15%] | {12 13 17} 42 [15%] |
| BLA | {15 21 15} 51 [29%] | {33 21 20}74 [43%] | {22 10 17} 49 [28%] |
| CeA + BLA | 249 (54%) | 117(26%) | 91(20%) |
Percent inside of brackets were calculated as of total for every amygdala region; i.e. CeA or BLA. Percent inside parenthesis were calculated as of total of projection targets; i.e. vlPAG, mPFC or vlPAG + mPFC. Numbers inside big parenthesis represented the cell counts from each individual mouse.
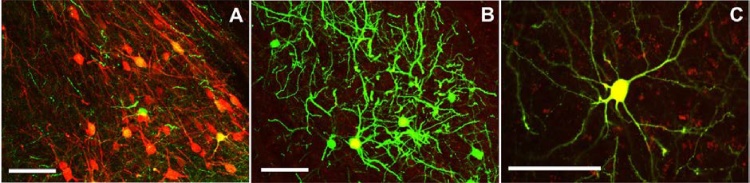
In either the CeA (Fig. 3C and 4A) or in the BLA (Fig. 3F, 4B and 4C) we also observed many retrograde double labeled (tdTomato+/GFP + cells) neurons indicating simultaneous projections to the vlPAG and the mPFC (Fig. 3F). In the CeA, around 15% of all retrograde labeled neurons connected down to the vlPAG and also sent a second axonal branch up to the mPFC. In the BLA, around 28% of neurons showed the same type of bifurcating projection. Altogether 20% of all retrograde labelled neurons found in the amygdala had bifurcating axons connecting simultaneously to the vlPAG and mPFC (Fig. 4).
Fig. 4.
Higher magnification pictures of CeA (A) and BLA (B) areas from Fig. 3C and 3 F, respectively. A typical bifurcating neurons from BLA is shown in C. Scale bar = 100 μm.
4. Discussion
Traditional retrograde tracers such as Fluro-Gold and CTb (cholera toxin B subunit), have been used to map brain neuronal circuits. Given the low efficiency and lack of specificity on labelling with these traditional tracers, various virus vector-based retrograde tracers were developed featuring higher tracing efficiency as well as the capacities to carry a fluorescent marker and/or to express specific peptides (Tervo, Hwang et al. 2016, Sun and Schaffer 2018). AAV-retro, a retrograde tracer based on recombinant AAV serotype 2 (rAAV2-retro), can label projecting neurons with fluorescent proteins like mCherry or GFP efficiently. Moreover, these new vectors are able to express distinct fluorescent reporters in either a generic mode (CAG promoter) or in a Cre-dependent mode (FLEX system). The pAAV-FLEX-tdTomato tracer used in this study expresses tdTomato in the presence of Cre. When injected into the vlPAG of the VGAT-Cre mice, AAV-retro viral particles traveled retrogradely toward the somata of amygdala neurons but only expressed tdTomato in the perikaryon of those neurons expressing Cre under VGAT promoter; i.e. GABAergic neurons. The other AAV-retro tracer pAAV-CAG-GFP, injected into the mPFC, expressed GFP in all kind (nonspecific expression) of amygdala neurons projecting to the mPFC. Thus under our labeling strategy TdTomato/GFP double labelling revealed bifurcating GABAergic neurons simultaneously projecting to both vlPAG and mPFC.
It is very well established that the amygdala has widespread projections connecting it with distant brain regions like the cerebral cortex, hypothalamus, and brainstem (Sah, Faber et al. 2003). Previous studies showed that the projections from the amygdala to brainstem largely originate from CeA GABAergic neurons while projections to the cerebral cortex mainly come from basal and lateral amygdala (Marek, Strobel et al. 2013, Li and Sheets 2018). In agreement with those studies, here we have confirmed that the large majority (71%) of amygdala neurons projecting to the midbrain reside in the CeA area. Also, and in agreement with those studies, we corroborated that up to 59% of amygdala neurons projecting to the mPFC are located in the BLA (the 41% remaining are in the CeA). We also found a non-small percent of BLA neurons projecting down to the vlPAG (29%). Because all these labeled neurons expressed tdTomato we conclude that, regardless of the location of their somata; i.e. CeA or BLA, these amygdala innervations to the vlPAG are GABAergic. In summary we found the connection from the amygdala to the vlPAG comes chiefly from the CeA, while the connection to the mPFC, arises roughly balanced between the BLA and the CeA.
Most notably we also found a number of amygdala neurons that had bifurcating axons connecting their somata simultaneously to the vlPAG and the mPFC. This is the first demonstration of the existence of such bifurcating GABAergic neurons in the amygdala. Because of the presence of tdTomato we know that, in the CeA, the bifurcating neurons made up 17.5% of all GABAergic neurons connecting with the vlPAG. Surprisingly, 40% of the BLA neurons projecting to mPFC are GABAergic bifurcating neurons sending also a second branch to connect with the vlPAG. Thus assessed by the relative abundance of the bifurcating neurons it suggested that the connectivity between BLA on one side, and the mPFC and the vlPAG on the other, is stronger than that showed between the CeA and its projection targets. In other words, the level of integration of the BLA with the vlPAG and mPFC seems to be tighter thanks to a larger contribution from bifurcating GABAergic neurons.
The mPFC is key to modulating decision making whereas the vlPAG is important to modulate defensive response. We propose that bifurcating GABAergic amygdala neurons either in the BLA or the CeA will facilitate the swift integration of emotional, cognitive and motor information. This quick integration will happen because information is arriving to the mPFC and vlPAG from the same amygdala neuron and at the same time. Fast integration of two function modalities by bifurcating amygdala neurons can produce a more adaptive emotional response.
Cataplexy, a sudden muscle atonia during waking, is a characteristic symptom of sleep disorder narcolepsy due to the loss of neuropeptide orexin (Chemelli, Willie et al. 1999). The neural mechanism mediating cataplexy is not fully understood. Emotion-induced cataplexy could be triggered by activating amygdala GABA neurons but the neural pathways involved have not been fully verified. Orexin neurons may use serotonin neurons in the dorsal raphe nucleus as a downstream target, to inhibit cataplexy by reducing the activity of amygdala as a center for emotional processing (Hasegawa, Maejima et al. 2017).Based on the facts that the vlPAG GABA neurons maintains muscle tone (Weber, Hoang Do et al. 2018) while mPFC is activated during emotions we propose here that the hyperactive GABAergic bifurcating neurons in amygdala might play a critical role on triggering cataplexy by sending inhibitory information downstream and upstream simultaneously during narcolepsy.
5. Conclusions
We found a group of bifurcating GABAergic neurons in the amygdala which project to both brain stem and cortex. Bifurcation might be a prevalent circuitry style in the central nervous system for integrating various behavioral processes.
Funding
Supported by NIH grants NS096151, NS101469, NS052287, NS079940, NS098541, DK115933 and DK084052 and Medical Research Service of the Department of Veterans Affairs.
Conflict of Interest
The authors declare no conflict of interest.
References
- Bienvenu T.C., Busti D., Micklem B.R., Mansouri M., Magill P.J., Ferraguti F., Capogna M. Large intercalated neurons of amygdala relay noxious sensory information. J. Neurosci. 2015;35(5):2044–2057. doi: 10.1523/JNEUROSCI.1323-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Naito J., Chen Y. Retrograde tracing with fluorescent microspheres reveals bifurcating projections from central retina to tectum and thalamus in chicks. Anat. Histol. Embryol. 2012;41(4):306–310. doi: 10.1111/j.1439-0264.2011.01131.x. [DOI] [PubMed] [Google Scholar]
- Chemelli R.M., Willie J.T., Sinton C.M., Elmquist J.K., Scammell T., Lee C., Richardson J.A., Williams S.C., Xiong Y., Kisanuki Y., Fitch T.E., Nakazato M., Hammer R.E., Saper C.B., Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98(4):437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Doron N.N., Ledoux J.E. Cells in the posterior thalamus project to both amygdala and temporal cortex: a quantitative retrograde double-labeling study in the rat. J. Comp. Neurol. 2000;425(2):257–274. [PubMed] [Google Scholar]
- Gibson D.A., Ma L. Developmental regulation of axon branching in the vertebrate nervous system. Development. 2011;138(2):183–195. doi: 10.1242/dev.046441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa E., Maejima T., Yoshida T., Masseck O.A., Herlitze S., Yoshioka M., Sakurai T., Mieda M. Serotonin neurons in the dorsal raphe mediate the anticataplectic action of orexin neurons by reducing amygdala activity. Proc. Natl. Acad. Sci. U. S. A. 2017;114(17):E3526–E3535. doi: 10.1073/pnas.1614552114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof P.R., Y.W, Bloom F.E., Belichenko P.V., Celio M.R. Elsevier; Amsterdam; New York: 2000. Comparative cytoarchitectonic atlas of the C57BL 6 and 129 Sv mouse brains. [Google Scholar]
- Katoh Y.Y., Benedek G. Cerebellar fastigial neurons send bifurcating axons to both the left and right superior colliculus in cats. Brain Res. 2003;970(1-2):246–249. doi: 10.1016/s0006-8993(03)02359-x. [DOI] [PubMed] [Google Scholar]
- Li J.N., Sheets P.L. The central amygdala to periaqueductal gray pathway comprises intrinsically distinct neurons differentially affected in a model of inflammatory pain. J. Physiol. 2018 doi: 10.1113/JP276935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek R., Strobel C., Bredy T.W., Sah P. The amygdala and medial prefrontal cortex: partners in the fear circuit. J. Physiol. 2013;591(10):2381–2391. doi: 10.1113/jphysiol.2012.248575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta S.C., Carobrez A.P., Canteras N.S. The periaqueductal gray and primal emotional processing critical to influence complex defensive responses, fear learning and reward seeking. Neurosci. Biobehav. Rev. 2017;76(Pt. A):39–47. doi: 10.1016/j.neubiorev.2016.10.012. [DOI] [PubMed] [Google Scholar]
- Rockland K.S. Collateral branching of long-distance cortical projections in monkey. J. Comp. Neurol. 2013;521(18):4112–4123. doi: 10.1002/cne.23414. [DOI] [PubMed] [Google Scholar]
- Rockland K.S. Corticothalamic axon morphologies and network architecture. Eur J. Neurosci. 2018 doi: 10.1111/ejn.13910. [DOI] [PubMed] [Google Scholar]
- Rushworth M.F., Behrens T.E. Choice, uncertainty and value in prefrontal and cingulate cortex. Nat. Neurosci. 2008;11(4):389–397. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- Sah P., Faber E.S., Lopez De Armentia M., Power J. The amygdaloid complex: anatomy and physiology. Physiol. Rev. 2003;83(3):803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Shiromani P.J., Floyd C., Velazquez-Moctezuma J. Pontine cholinergic neurons simultaneously innervate two thalamic targets. Brain Res. 1990;532(1-2):317–322. doi: 10.1016/0006-8993(90)91774-b. [DOI] [PubMed] [Google Scholar]
- Sun S., Schaffer D.V. Engineered viral vectors for functional interrogation, deconvolution, and manipulation of neural circuits. Curr. Opin. Neurobiol. 2018;50:163–170. doi: 10.1016/j.conb.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervo D.G., Hwang B.Y., Viswanathan S., Gaj T., Lavzin M., Ritola K.D., Lindo S., Michael S., Kuleshova E., Ojala D., Huang C.C., Gerfen C.R., Schiller J., Dudman J.T., Hantman A.W., Looger L.L., Schaffer D.V., Karpova A.Y. A designer AAV variant permits efficient retrograde access to projection neurons. Neuron. 2016;92(2):372–382. doi: 10.1016/j.neuron.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote P., Esposito M.S., Botta P., Chaudun F., Fadok J.P., Markovic M., Wolff S.B., Ramakrishnan C., Fenno L., Deisseroth K., Herry C., Arber S., Luthi A. Midbrain circuits for defensive behaviour. Nature. 2016;534(7606):206–212. doi: 10.1038/nature17996. [DOI] [PubMed] [Google Scholar]
- Weber F., Hoang Do J.P., Chung S., Beier K.T., Bikov M., Saffari Doost M., Dan Y. Regulation of REM and Non-REM Sleep by Periaqueductal GABAergic Neurons. Nat. Commun. 2018;9(1):354. doi: 10.1038/s41467-017-02765-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.Y., Liu T.H., He Y., Pan H.Q., Zhang W.H., Yin X.P., Tian X.L., Li B.M., Wang X.D., Holmes A., Yuan T.F., Pan B.X. Chronic stress remodels synapses in an amygdala circuit-specific manner. Biol. Psychiatry. 2019;85(3):189–201. doi: 10.1016/j.biopsych.2018.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]