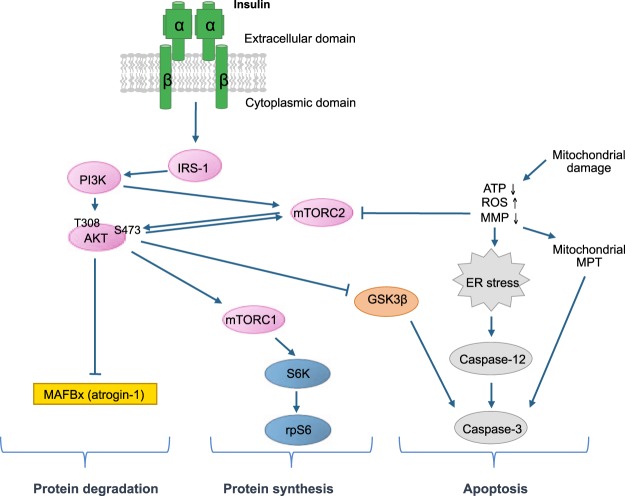
Figure 1.
Simplified representation of the IR/Akt/mTOR and related pathways. Upon binding of insulin to its receptor (IR), autophosphorylation and activation of the receptor occurs, leading to the translocation of Akt to the plasma membrane where it is phosphorylated at the Thr308 site by PI3K and at the Ser473 site by mTORC2. After full activation, Akt promotes protein synthesis via mTORC1 activation and prevents caspase activation by phosphorylating and thereby inhibiting glycogen synthase kinase (GSK) 3β. Activated Akt also inhibits protein degradation by repressing MAFBx mRNA expression. Mitochondrial damage is associated with a drop in the cellular ATP content, reactive oxygen species (ROS) production and a drop in the mitochondrial membrane potential (MMP). This leads to impaired activation of mTORC2 and activation of apoptosis via mitochondrial membrane permeability transition (MPT) and ER stress. While insulin inhibits apoptosis by activation of Akt, it can also increase ER stress in the presence of ER stress inducers and thereby stimulate cleavage of caspase-12.