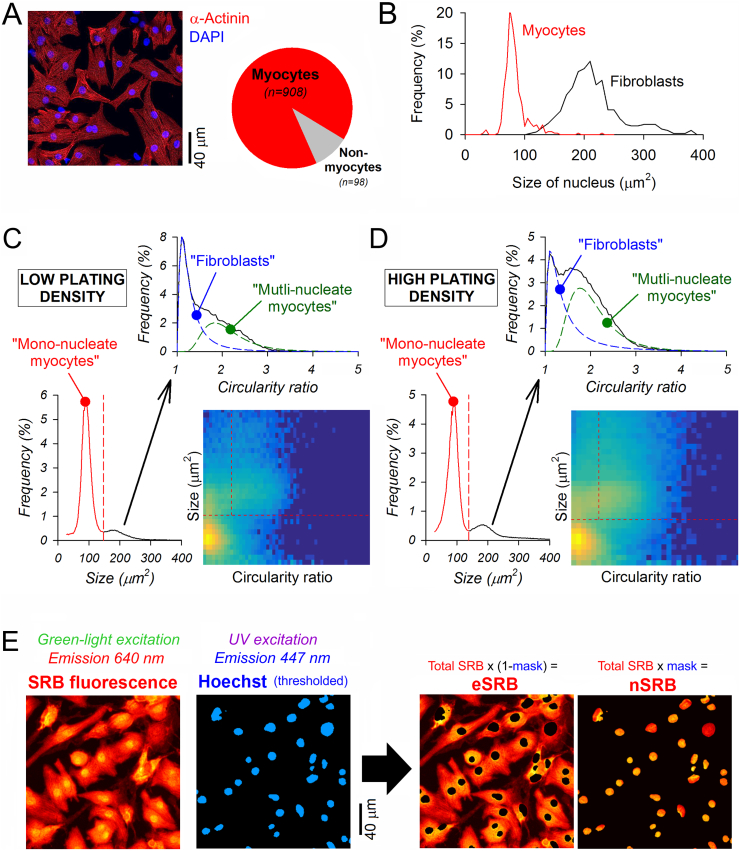
Fig. 2.
Workflow for determining counts of cells by sub-type and the subcellular distribution of SRB. (A) Immunofluorescence confocal image of NRVM monolayer stained for α-actinin (cardiomyocyte marker) and the nuclear stain DAPI. Based on an analysis of images from 14 cell isolations, ~90% of cells were identified as myocytes. (B) Statistical distribution of the area of Hoechst-positive particles detected in myocytes under normal NRVM culture, compared to fibroblast-enriched cultures obtained from the pre-plating step and imaged in separate experiments (from 4 isolations). Data obtained by high-magnification microscopy. (C) Analysis of nuclear particle geometry in NRVMs cultured at low density (~1500 particles/field of view); data pooled from 96 wells imaged by Cytation 5. Circularity ratio is the quotient of the long-axis to short-axis diameter of the particle. Analyzed by area, the majority of particles are representative of mononucleated myocytes. When plotted against circularity ratio, the population with larger nuclei can be described as the sum of two log-normal distributions representing fibroblasts and binucleated myocytes (dashed lines). (D) Analysis repeated on cells cultured at higher density (~3000 particles per field of view). (E) Sequential imaging of SRB and Hoechst. A binary mask, determined from the pattern of Hoechst staining, was used to segregate extra-nuclear and nuclear SRB signals (eSRB, nSRB).