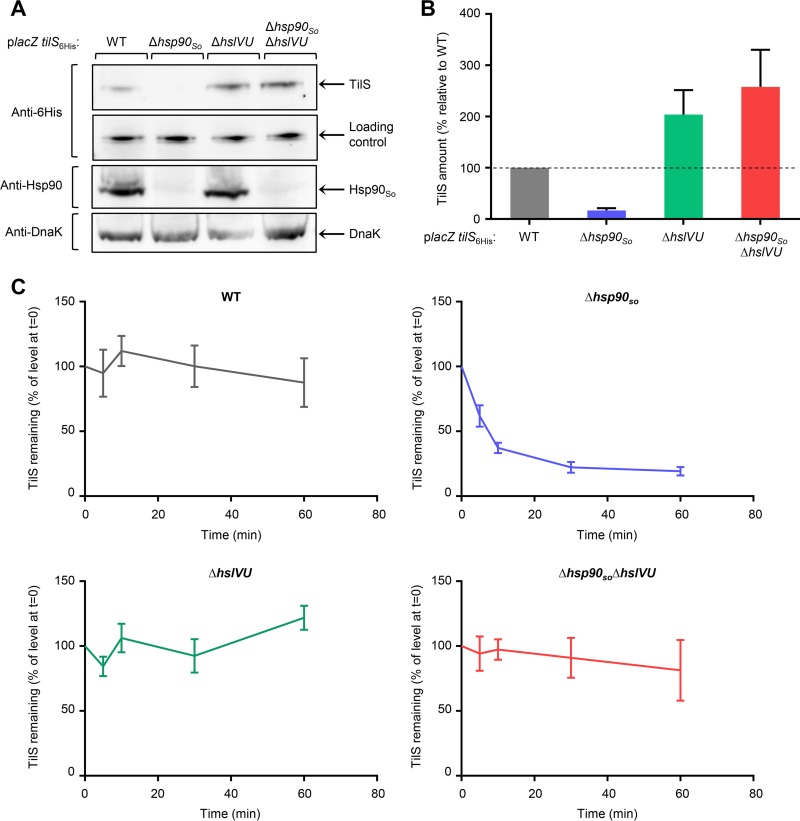
FIG 2.
The HslVU protease degrades the Hsp90So client TilS. (A) Strains containing the placZ tilS6His plasmid, in which lacZ and tilS6His are two independent genes under the control of the PBAD promoter, were grown at 35°C, a sublethal temperature. At an OD600 of 0.6, 0.02% arabinose was added. After 2 h at 35°C, the same amounts of total protein extract from each strain were loaded for SDS-PAGE, transferred by Western blotting, and revealed with anti-6× His antibody to detect the TilS protein, anti-Hsp90 antibody, or anti-DnaK antibody. The loading control corresponds to a contaminating band revealed with the anti-6× His antibody, indicating that the same amount of cellular extracts was loaded. (B) Quantification of the amount of TilS was performed from 3 independent Western blots described in panel A, revealed with the anti-6× His antibody using ImageJ software. The amount of TilS measured in the wild-type strain was set to 100%. Data are shown as mean ± SEM. (C) Chase experiments. Strains containing the placZ tilS6His plasmid were grown as in panel A, except that 0.2% arabinose was added to increase the level of the TilS protein, in particular in the Δhsp90So strain. After 2 h of induction, 200 μg/ml chloramphenicol was added to block protein translation (t = 0). Samples were taken at several times after chloramphenicol addition, and proteins were precipitated with trichloroacetic acid (TCA), loaded for SDS-PAGE, and quantified on a Western blot, revealed with anti-6× His antibody using the ImageJ software. The amount of TilS measured in each strain at t = 0 (chloramphenicol addition) was set to 100%. Data are shown as mean ± SEM.