Table 3.
TPPU and its identified Metabolites: chemical formula, structures, inhibitory potency toward human she, and non-compartmental pharmacokinetic parameters after oral gavage at a 10 mg/kg in rats (n = 4).
| Meta. ID | Formula | HsEH IC50a (nM) | AUCb (nM*h) | Cmaxc (nM) | T1/2d (h) |
|---|---|---|---|---|---|
| TPPU | 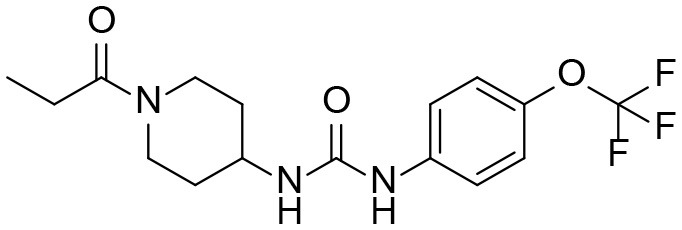 |
1.1 ± 0.1 | 6.0 ± 0.9 106 | 2.0 ± 0.3 105 | 9.0 ± 1.8 |
| 1-(1-propionylpiperidin-4-yl)-3-(4-(trifluoromethoxy)phenyl)urea | |||||
| M1 | 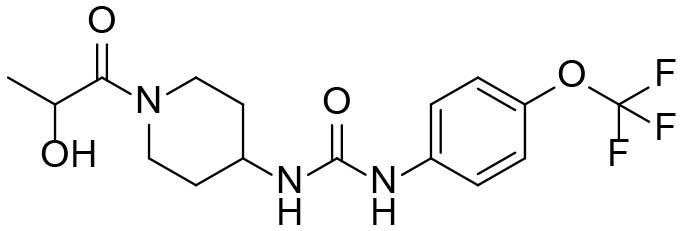 |
3 ± 0.5 | 6.0 ± 1.0 105 | 1.0 ± 0.4 104 | 20 ± 3 |
| 1-(1-(2-hydroxypropanoyl) piperidin-4-yl)-3-(4-(trifluoromethoxy)phenyl)urea | |||||
| M2 | 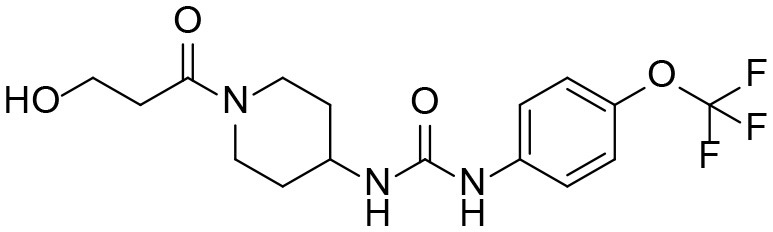 |
16 ± 2 | 2.0 ± 0.6 104 | 7.0 ± 2.0 102 | 9.7 ± 0.8 |
| 1-(1-(3-hydroxypropanoyl) piperidin-4-yl)-3-(4-(trifluoromethoxy)phenyl)urea | |||||
| M3 | 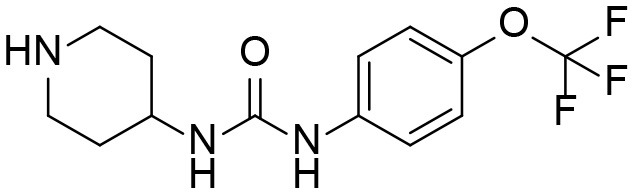 |
83 ± 9 | 6.0 ± 4.0 103 | 3.0 ± 2.0 102 | 14 ± 2.7 |
| 1-(piperidin-4-yl)-3-(4-(trifluoromethoxy)phenyl)urea | |||||
| M4 | 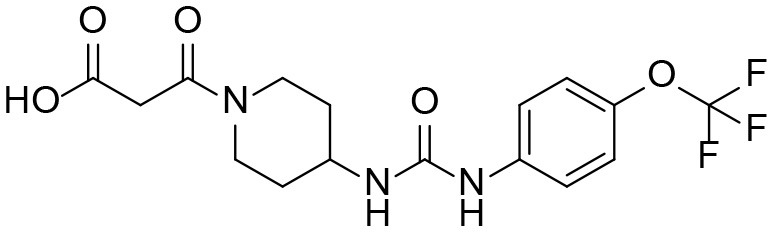 |
158 ± 10 | 1.0 ± 0.4 103 | 53 ± 28 | 17 ± 4.7 |
| 3-oxo-3-(4-(3-(4-(trifluoromethoxy)phenyl)ureido) piperidin-1-yl)propanoic acid |
IC50 values were determined by CMNPC fluorescent assay.
Area under the concentration (Time0−72h).
Maximum blood concentration.
half-life.
