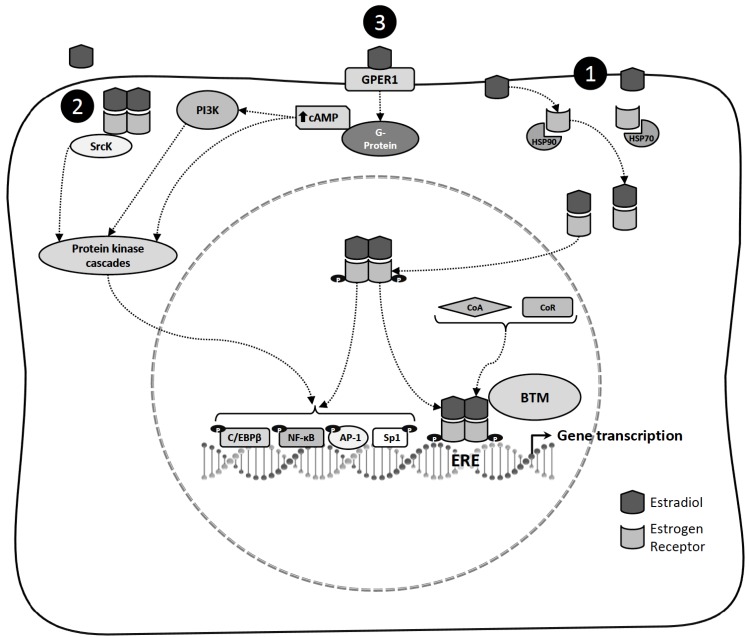
Fig. (1).
Mechanisms of estradiol action. 1) In the classical model of estradiol action, in the absence of ligand, estrogen receptor is associated with heat shock proteins (HSP70 and HSP90). Estradiol interaction induces estrogen receptor conformational changes that allow dissociation of the HSP, promoting dimerization, phosphorylation and high-affinity binding to estrogen response elements (ERE) located within the regulatory regions of target genes. Estrogen receptor modulates target gene transcription by recruiting components of the basal transcriptional machinery (BTM) and by interacting with coregulatory proteins (Coactivators: CoA or Corepressors: CoR). Estrogen receptor can also modulate the expression of genes without directly binding to deoxyribonucleic acid by tethering to other transcription factors through protein-protein interactions in the nucleus, including specificity protein 1 (Sp1) and activator protein 1 (AP-1), nuclear factor kappa B (NF-ҡB) and CCAAT/enhancer binding protein β (C/EBPβ) to regulate gene promoters that lack canonical ERE sequences. 2) Estrogen receptor localized into the cell membrane or cytoplasm has been proposed to mediate estradiol activation of the protein tyrosine Kinase (SrcK)/mitogen-activated protein kinase (MAPK) signaling pathway, and can phosphorylate and active certain nuclear transcription factors. 3) In the non-classical mechanisms of estradiol action, estradiol binding to G protein-coupled estrogen receptor-1 (GPER1) induces cyclic adenosine monophosphate (cAMP) production and MAPK, and activates phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathways.