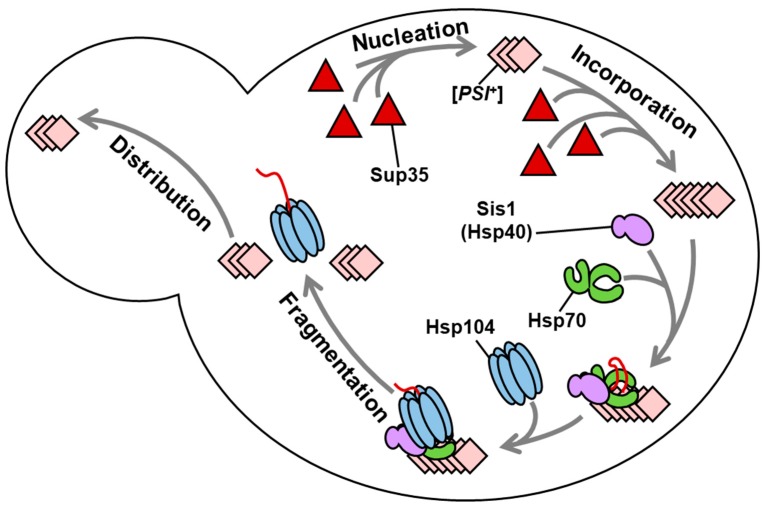
Figure 1.
Current model of chaperone-dependent prion propagation in vivo. Four interdependent processes are necessary for prion formation and propagation in yeast. Prions arise from a rare event in which protein monomers (represented as triangles in the case of the prion-forming protein Sup35 above) misfold and form a thermodynamically-stable, ordered aggregate (“nucleation”). Aggregates increase in size by recruiting more soluble monomer (“incorporation”). To be transmissible, however, the fibril must also be a target for the chaperone machinery [2]. Hsp40-class chaperones, particularly Sis1, and the Hsp70 Ssa, are thought to functionally recruit the disaggregase Hsp104, responsible for the physical fragmentation of amyloid fibrils to create new propagons (“fragmentation”) that may be inherited by daughter cells (“distribution”).