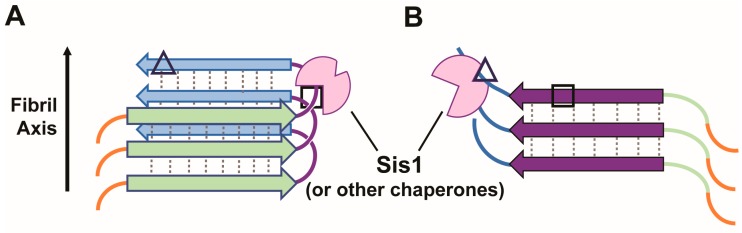
Figure 2.
Prion variants may expose or obscure binding sites for distinct chaperone domains. Examples of two prion variants (“A” and “B”) in which different regions form the amyloid core, assumed here to be in-register parallel beta-sheet. The structural basis of yeast prion amyloids is debated, as is the structural nature of variants. See Wickner et al. for a recent review [99]. The solid arrow represents the direction of growth of the amyloid fibril, while dashed lines represent hydrogen bonds between parallel, in-register β-strands (horizontal arrows) that restrict the binding of chaperones to sites within the amyloidogenic regions. These sites (triangle and square) vary in structure when exposed and therefore may be recognized by distinct binding modes or domains of Sis1 and/or other chaperone proteins (pink cartoon). Thus, binding occurs only when the site is exposed, i.e., not part of the amyloid core.