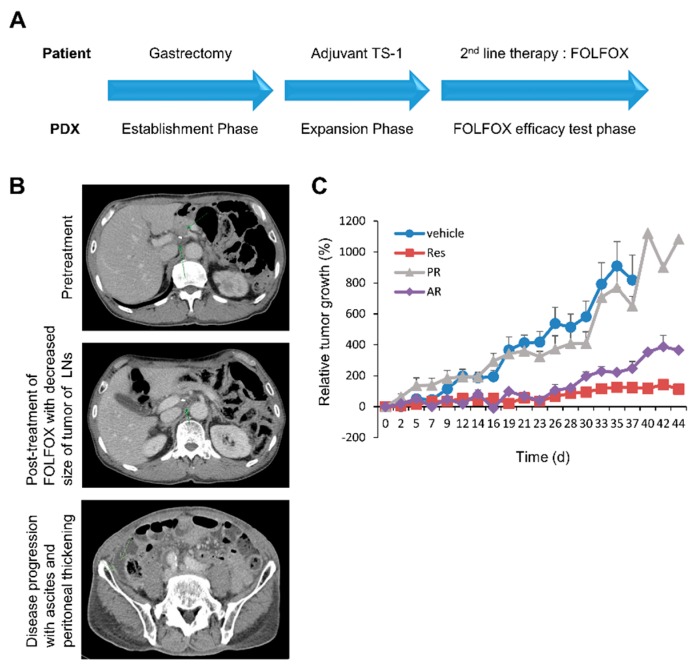
Figure 2.
The poor clinical outcome of GC patient, and the heterogeneous response of patient-derived xenograft (PDX) tumors to 5-fluorouracil and oxaliplatin modulated with leucovorin (FOLFOX). (A) Establishment and treatment of PDX tumors simultaneously with the clinical course of the patient. After gastrectomy, the primary tumor of the patient was engrafted into nude mice and allowed to expand for a FOLFOX-efficacy test. The patient in whom the adjuvant TS-1 chemotherapy has failed was administered into second-line therapy with FOLFOX accompanied by PDX. (B) CT images taken during the clinical course of the patient. The recurrent tumors (upper panel, arrow) showed a reduction in size after FOLFOX chemotherapy (middle panel, arrow). Subsequently, the peritoneal metastasis (lower panel, left arrow) grew, and ascites (lower panel, right arrow) developed. Tumor-bearing immunodeficient mice (n = 8 vehicle and n = 9 FOLFOX mice in each treatment group) were treated when the tumor had reached a volume of 100–200 mm3. FOLFOX (5-fluorouracil at 15 mg/kg and oxaliplatin at 5 mg/kg) was administered as described in the Methods section. (C) The reanalyzed tumor growth kinetics (n = 9 vehicle, n = 3 Res, n = 3 PR and n = 2 AR) from the FOLFOX-treatment experiment (Figure S3a).