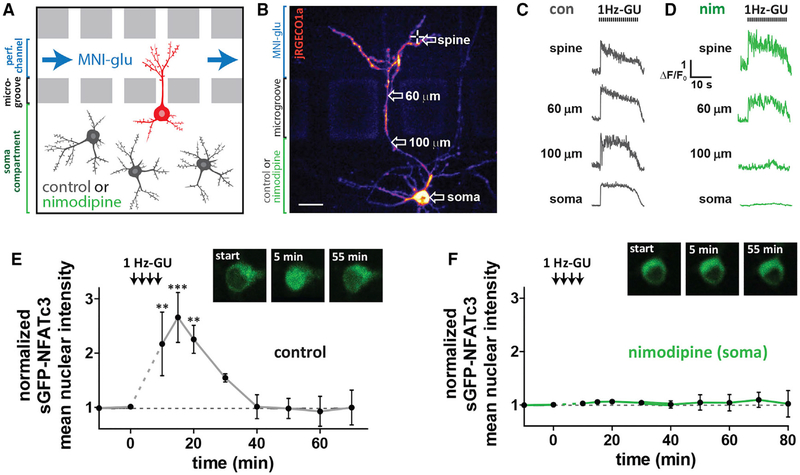
Figure 5. Selective Block of Somatic LTCCs Prevents NFATc3 Translocation Following Distal Spine Stimulation.
(A) Schematic of neurons grown in microfluidic culture device. The perfusion channel and somatic compartment are pharmacologically isolated due to hydrostatic pressure across microgrooves preventing solution exchange. LTCC antagonist nim (5 μM) or control solution were added to the soma compartment and MNI-glu and cy5.5 were added to the perfusion channel.
(B) Cultured hippocampal neuron grown in a microfluidic device and transfected with jRGECO1a (pseudocolored) and sGFP-NFATc3. Scale, 20 μm.
(C) jRGECO1a ΔF/F0 traces from control neuron taken at various distances from uncaged spine following 1 Hz-GU.
(D) jRGECO1a ΔF/F0 traces from neuron in (B) with nim (5 μM) in soma compartment.
(E) sGFP-NFATc3 nuclear intensity following distal spine uncaging for neuron grown in a microfluidic device with control solution in soma compartment (n = 3; one-way ANOVA repeated-measures with Dunnett, ***p < 0.001, **p < 0.01, *p < 0.05). Inset: images of soma showing sGFP-NFATc3 distribution.
(F) As in (E) but with nim in soma compartment (n = 5). Inset: image of soma showing sGFP-NFATc3 distribution for neuron in (B).
See also Figure S5.