Fig. 7.
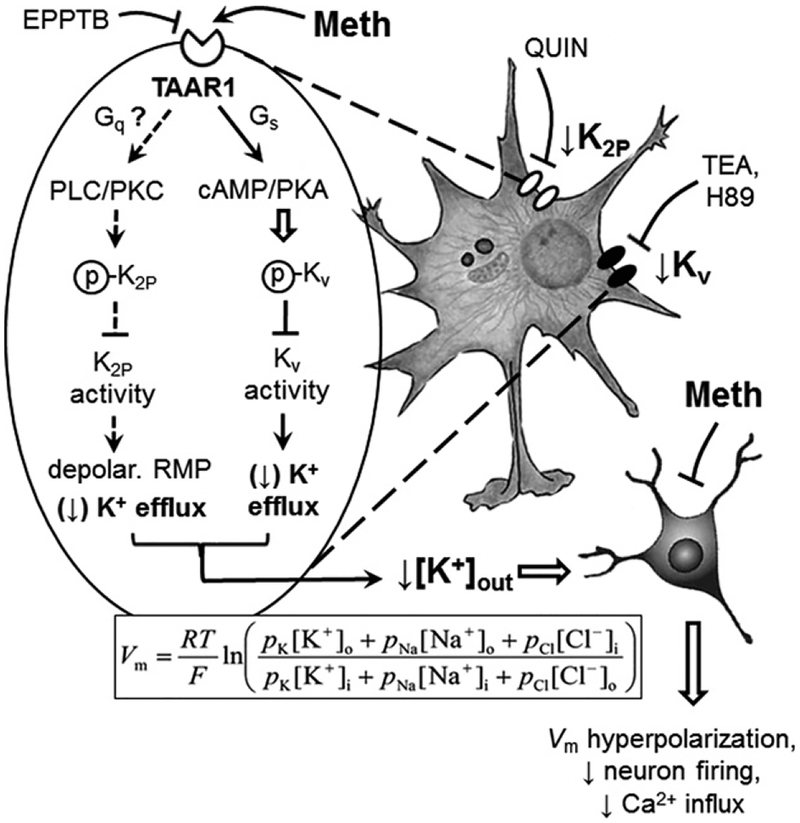
Acute methamphetamine (Meth) effects on altering astrocyte activity are mediated by TAAR1-coupled signaling and K+ channel dysfunction. Astrocytes play a key role in regulating extracellular K+ homeostasis in the brain. Meth alters this dynamic function of astrocytes in regulating K+ homeostasis by suppressing K+ efflux through dysfunctional Kv, K2P and Kir channels. Acute Meth effects on altering K+ efflux through Kv channels are mediated by disrupting the TAAR1-coupled cAMP/PKA signaling pathway. The mechanism underlying acute Meth effects on suppressing K2P channel activity [leading to resting membrane potential (RMP) depolarization] could be mediated by dysfunction of the Gs/cAMP/PKA and/or Gq/phospholipase C (PLC)/protein kinase C (PKC) signaling pathway. Meth is a potent agonist for TAAR1. Direct activation of TAAR1 by Meth promotes the TAAR1/Gs/cAMP/PKA signaling pathway, as well as the TAAR1/Gq/PLC/PKC signaling pathway, thereby enhancing PKA/PKC-induced phosphorylation of Kv/K2P channels, respectively. Enhanced phosphorylation of Kv/K2P channels reduces their activity to conduct outflowing K+. Reducing K+ efflux from astrocytes via Kv/K2P channels decreases extracellular K+ levels ([K+]o). The Goldman-Hodgkin Katz equation indicates that reduced [K+]o results in Vm hyperpolarization of surrounding neurons; and therefore decreases their excitability. The mechanism, by which toxic/fatal level of Meth suppresses Kir channel activity, remains to be identified. These findings suggest that Meth disturbs functional and dynamic activity of astrocytes by altering the functional activity of Kv and K2P channels through the cAMP/PKA and likely PLC/PKC signaling pathway, respectively; and that could affect their interactions with neurons in the brain.