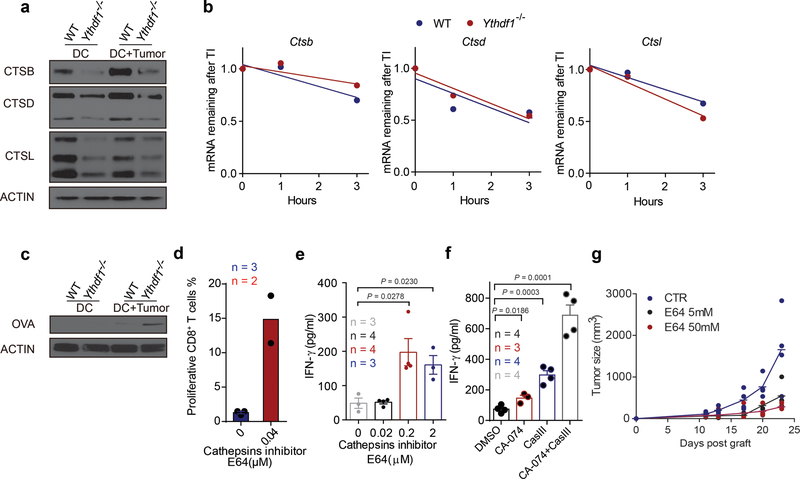
Extended Data Fig. 8 |. Antigen degradation is reduced in Ythdf1−/− mice and inhibition of protease cathepsins enhanced the cross-priming of WT DCs.
a, GMDCs were co-cultured with necrotic B16-OVA cells overnight. Immunoblot analysis of proteases Cathespins B/D/L (CTSB, CTSD and CTSL) in GMDCs. b, WT and Ythdf1−/− DCs were treated with Actinomycin D, RNAs collected at different time points after treatment, and mRNA levels were measured using RT-qPCR and represented as mRNA remaining after transcription inhibition (TI). ns not significant. c, GMDCs were co-cultured with necrotic B16-OVA cells overnight and OVA degradation in BMDCs was measure by Immunoblot. d, Ex vivo purified wild-type cDCs were pre-treated with 0.04 μM cathepsin inhibitor E64 and pulsed with OVA protein for 4 h. The cross-priming capacity of DCs was compared by co-culturing DCs with cell trace violet (CTV) labeled OTI-T cells. The proliferation was measured by the dilution of CTV. e, GMDCs were pre-treated with 0.2–2 μM cathepsin inhibitor E64 and co-cultured with B16-OVA cells. The cross-priming capacity of DC was compared by co-culturing DCs with isolated CD8+ T cells from naive OTI mice and analyzed by IFN-γ cytometric bead array. f, Flt3L-DCs were pre-treated with cathepsin inhibitor CA-074 or/and cathepsin L inhibitor III (CASIII), followed by co-culturing with necrotic B16-OVA cells. Synergistic inhibition effects were observed. The cross-priming capacity of DC was determined. g, data points for Fig. 4b. n = 3 independent experiments with similar results (a, c); n = 2 independent experiments (b). n, numbers of sample size. Data are mean ± s.e.m. and were analyzed by two-tailed unpaired Student’s t-test (e) or one-tailed unpaired Student’s t-test (f).