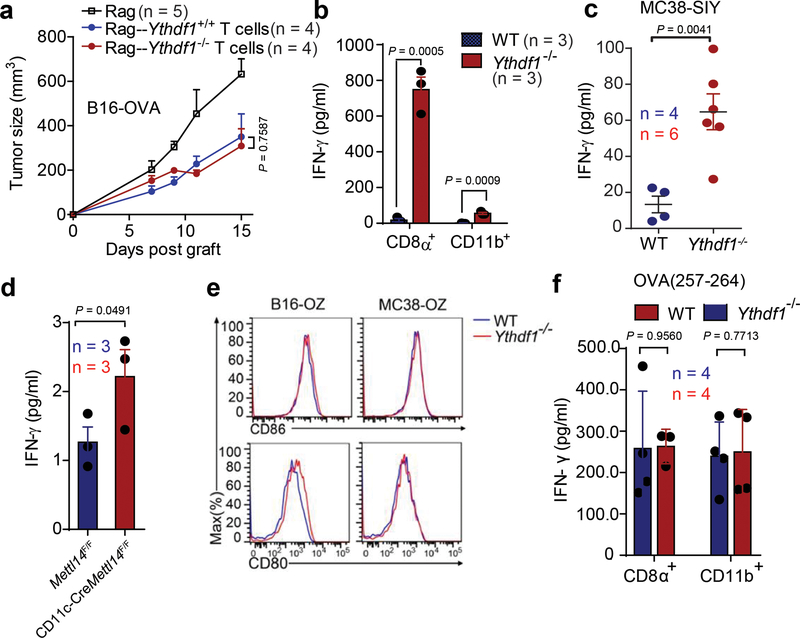
Extended Data Fig. 3 |. Cross priming of tumor neoantigen is increased in Ythdf1-deficient mice.
a, Rag2−/− mice were transferred with T cells isolated from WTor Ythdf1−/− miceon day 0. On the same day, mice were injected s.c. with 5×105 B16-OVA cells. Tumor growth was monitored over time. b, WT or Ythdf1−/− mice were injected s.c. with 106 MC38-OTIp cells. 6 days after tumor inoculation, CD8+ or CD11b+ DCs were sorted from draining LNs. DCs were co-cultured with CD8T+ cells isolated from naive OTI mice. Capacity of cross priming was determined by the production of IFN-γ. c, WT or Ythdf1−/− mice were injected s.c. with 106 MC38-SIY cells. 6 days after tumor inoculation, DCs were sorted from draining LNs and co-cultured with CD8+ T cells isolated from naive 2C mice. Capacity of cross priming was determined by the production of IFN-γ. d, WT or Mett14-deficient GMDCs were co-cultured with B16-OVA cells. The cross-priming capacity was shown. e, WT or Ythdf1−/− mice were injected s.c. with 106 B16-OVAcells. Data is shown as the expression of CD80 and CD86 on tumor infiltrating DCs. f, WT or Ythdf1−/− mice were injected s.c. with 106 B16-OVA cells. 6 days after tumor inoculation, CD8+ or CD11b+ DCs were sorted from draining LNs. DCs were pulsed with 1 μg/ml exogenous OT-I peptide and co-cultured with isolated CD8+ T cells from naive OTI mice for 3 days and analyzed by IFN-γ CBA. Data are representative of two independent experiments with similar results(e). n, numbers of mice. Data are mean ± s.e.m. and were analyzed by two-tailed unpaired Student’s t-test (a-c, f) or one-tailed unpaired Student’s t-test (d).