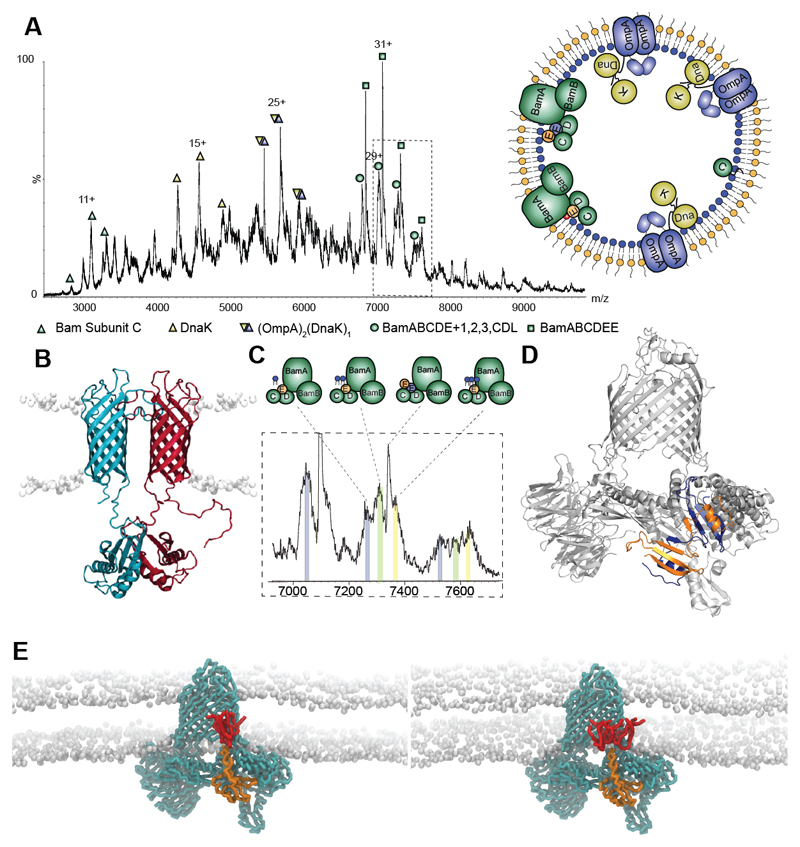
Fig. 1. Protein complexes ejected directly from E. coli outer membranes.
A Mass spectrum recorded at 400 V assigned to BamC, DnaK, DnaK:OmpA:pro-OmpA, and two states of the Bam complex with inset an outer membrane vesicle with complexes observed. B Model of the OmpA dimer (15) with the hydrophobic pro-sequence (red) a potential binding site for DnaK. C Expansion of the mass spectrum assigned to the Bam complex with monomeric BamE (Bam ABCDE) binding to one, two and three cardiolipins (grey, green, yellow respectively). D Atomic structure of BamE dimer (PDB: 2YH9) (orange and blue) docked into the Bam complex (PDB: 5D0O) with BamE monomer removed. E MD simulations of the BamABCDE complex (cyan) with monomeric BamE (orange) and two (lhs) and three (rhs) CDL molecules (red).