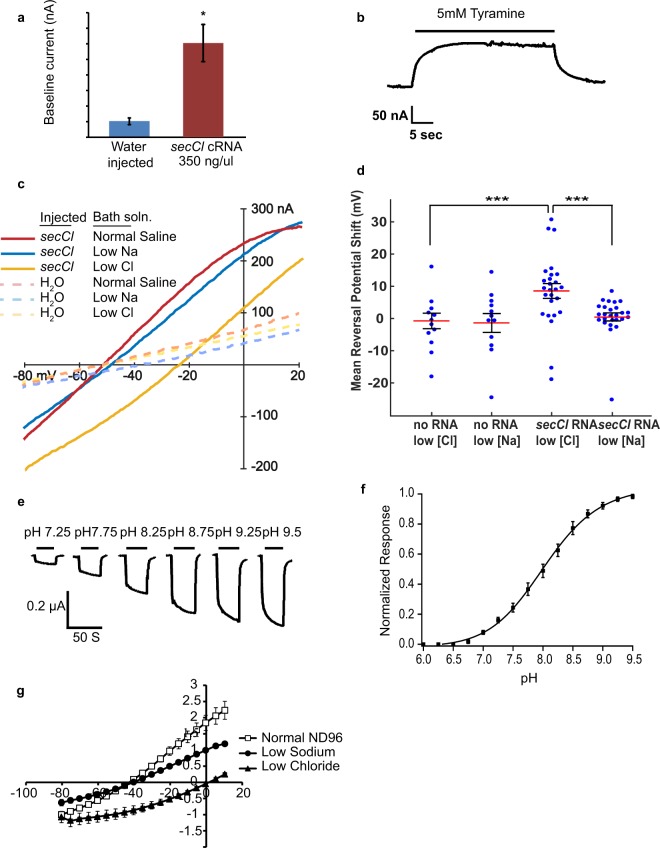
Figure 1.
secCl forms a constitutively open homomeric chloride channel. (a) Bars indicate baseline currents of oocytes clamped at −80 mV that were injected with either water (n = 5) or secCl cRNA (n = 10). Error bars represent standard error of the mean. *P < 0.002 (two-tailed t-test). (b) Sample trace from an oocyte clamped at −80mV expressing secCl. The upward deflection corresponds to channels closing or being blocked upon treatment with 5 mM tyramine. (c) Sample current-voltage (IV) curves from an oocyte injected with secCl cRNA (solid traces) and a control oocyte injected with water (dashed traces). “Normal Saline” (ND96: 96 mM Na+ and 103.6 Cl−) “low Na” (6 mM Na+ and 103.6 mM Cl−) and”Low Cl” (96 mM Na+ and 13.6 mM Cl−). (d) Reversal potential shifts of secCl-expressing and control oocytes in low-chloride and low-sodium buffers relative to normal saline. ***P < 0.0001. (e–g) Coexpression of secCl with CG6927 results in a heteromeric pH-sensitive chloride channel. (e) Representative traces from an oocyte clamped at −80 mV expressing secCl and CG6927, showing responses to changes in pH. Control buffer was ND96 at pH 6.0. (f) The response profile of the channel to changes in pH. The responses were normalized to the maximum current response of each oocyte. The curve represents the fit to the Hill equation (n = 5). (g) Representative traces of the current voltage relationship in “normal” (n = 6), “low sodium” (n = 4), and “low chloride” (n = 4) buffer. Error bars represent standard error of the mean.